Programmed death receptor 1 gene inhibitor and its preparation method and application
A programmed death and receptor technology, applied in the direction of non-central analgesics, anti-inflammatory agents, antiviral agents, etc., can solve the problems of high storage conditions, unfavorable transportation and storage, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
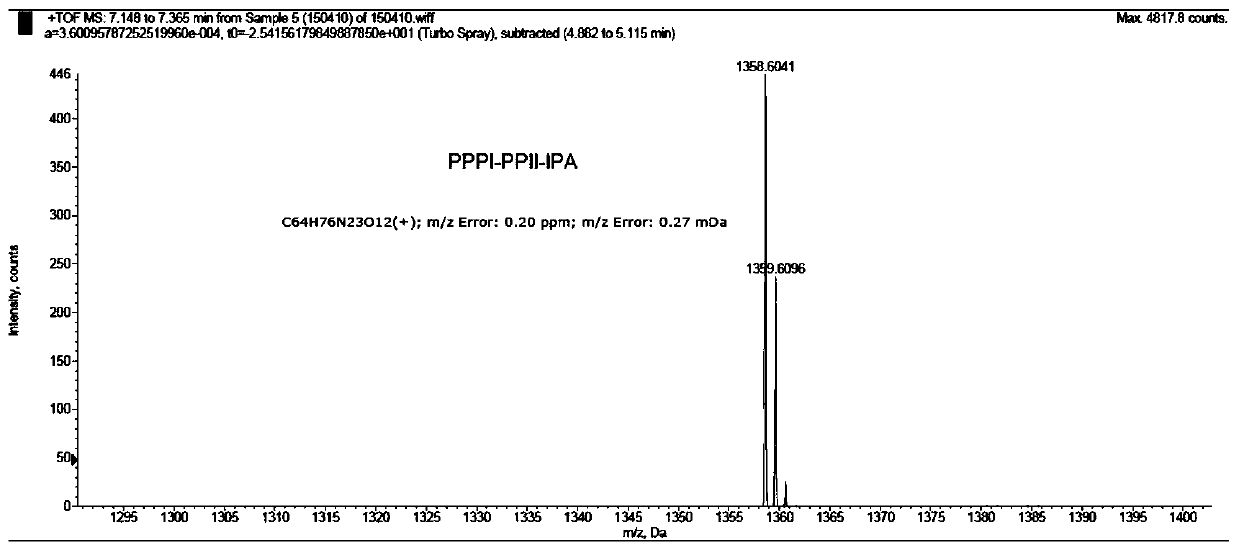
Embodiment 1
[0076] The preparation method of embodiment 1 compound 1A
[0077] (a) Resin swelling: Add 400mg Fmoc-protected phenylhydrazine resin (0.66mmol / g, 0.264mmol) and 3mL CH to a 10mL solid phase reactor 2 Cl 2 , the resin was swollen for 30min, and the CH 2 Cl 2 ,spare;
[0078] (b) Remove the Fmoc protecting group: add 3mL of 20% piperidine / DMF solution to the resin after step (a) swelling, N 2 Bubble and mix well, after 10min, remove the solvent, then add 3mL 20% piperidine / DMF solution, N 2 Bubble and mix well, after 10min, wash the resin with DMF (4×3mL), then wash the resin with 3mL anhydrous DMF, set aside;
[0079](c) Amino acid condensation: 4-tert-butoxycarbonylamino-1-methyl-1H-pyrrole-2-carboxylic acid (254 mg, 1.056 mmol) and triphosgene (BTC, 128 mg, 0.433 mmol) were dissolved in 2 mL of anhydrous THF, slowly add collidine (collidine, 488μL, 3.696mmol) dropwise to the solution, the reaction immediately produces a large amount of white precipitate, after adding t...
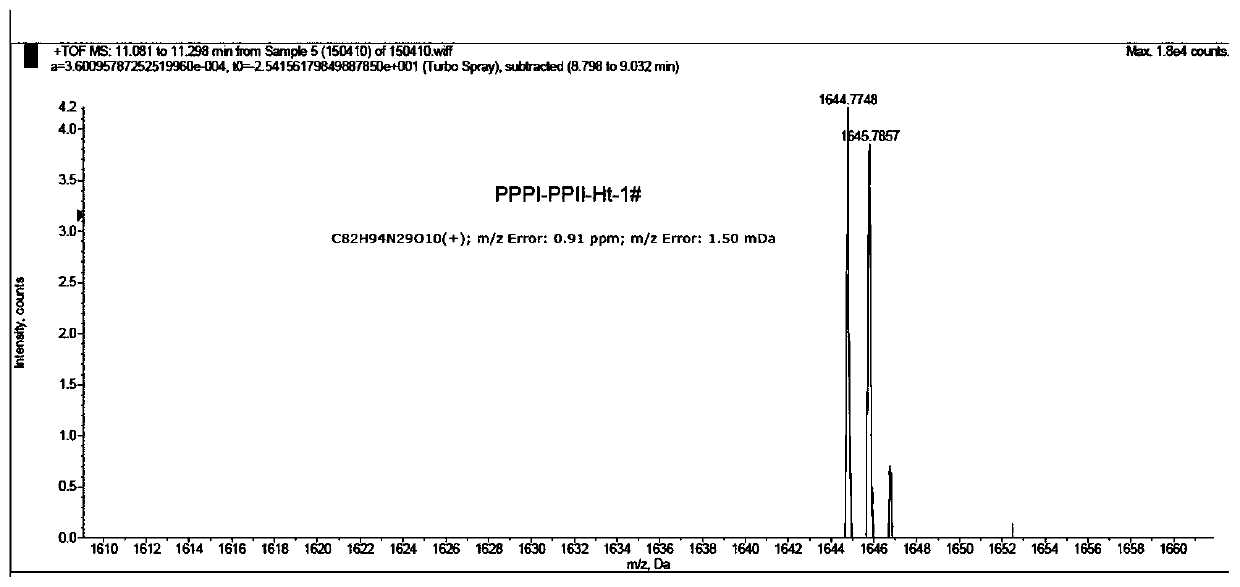
Embodiment 2
[0101] The preparation method of embodiment 2 compound 1B
[0102] The peptide loaded on the phenylhydrazine resin shown in formula (7) was prepared in the same steps as in Example 1,
[0103]
[0104] Take out the resin obtained above, add 1mL DMF, 200μL N,N-bis(3-aminopropyl)methylamine, shake at 90°C for 1h, cool to room temperature, filter the resin, and wash with 20mL CH 2 Cl 2 The resin was washed; the organic phase was concentrated and the residue was purified by semi-preparative HPLC: 10% acetonitrile-H 2 O (containing 1% TFA) isocratic elution 5min, 10% to 100% acetonitrile-H 2 O (containing 1% TFA) gradient elution for 25min, retention time T R =16min, the product was collected and freeze-dried to obtain a light yellow solid, which was set aside;
[0105] Dissolve 4 mg (3 μmol) of the above solid in 0.5 mL of anhydrous DMF, add Horst acid derivative Ht-2 (2.7 mg, 6 μmol), PyBOP (3.1 mg, 6 μmol) and DIEA (5 μL, 30 μmol), shake at room temperature Reaction 2h, ...
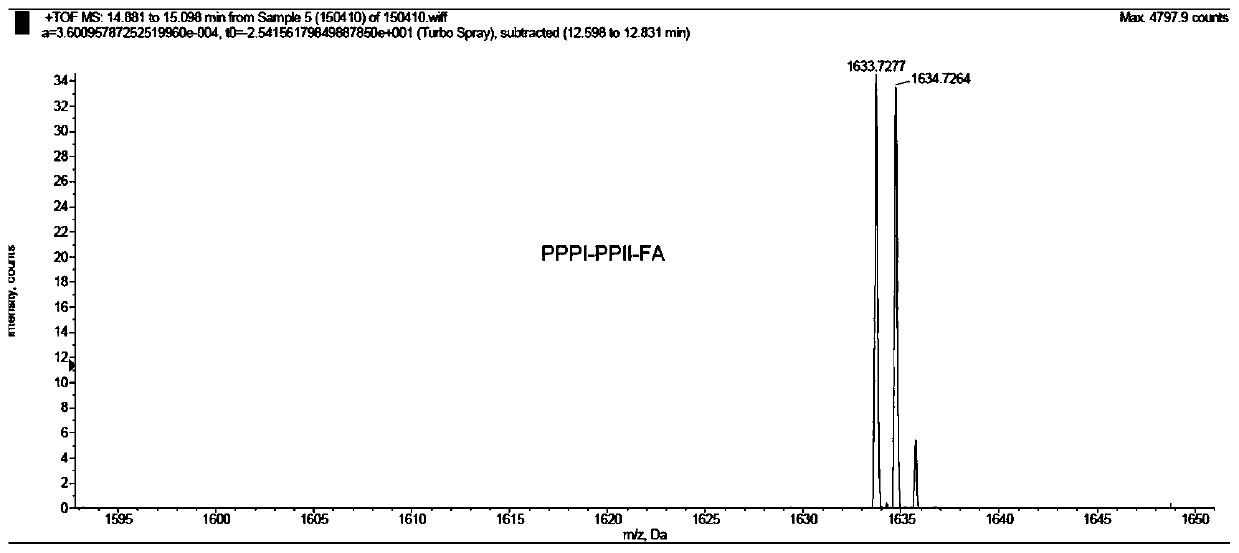
Embodiment 3
[0113] The preparation method of embodiment 3 compound 1C
[0114] The peptide loaded on the phenylhydrazine resin shown in formula (7) was prepared in the same steps as in Example 1,
[0115]
[0116] Take out the resin obtained above, add 1mL DMF, 200μL N,N-bis(3-aminopropyl)methylamine, shake at 90°C for 1h, cool to room temperature, filter the resin, and wash with 20mL CH 2 Cl 2 The resin was washed; the organic phase was concentrated and the residue was purified by semi-preparative HPLC: 10% acetonitrile-H 2 O (containing 1% TFA) isocratic elution 5min, 10% to 100% acetonitrile-H 2 O (containing 1% TFA) gradient elution for 25min, retention time T R =16min, the product was collected and freeze-dried to obtain a light yellow solid, which was set aside;
[0117] Dissolve 4 mg (3 μmol) of the above solid in 0.5 mL of anhydrous DMF, add folic acid FA (1.8 mg, 6 μmol), PyBOP (3.1 mg, 6 μmol) and DIEA (5 μL, 30 μmol), shake at room temperature for 2 h, use semi-preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com