Lupeol derivative as well as synthesis method and application thereof
A technology for lupin alcohol and derivatives, which is applied in the field of biosynthesis, can solve the problems of restricted drug screening, less oxidation sites of lupin alcohol parent nucleus, difficult chemical synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
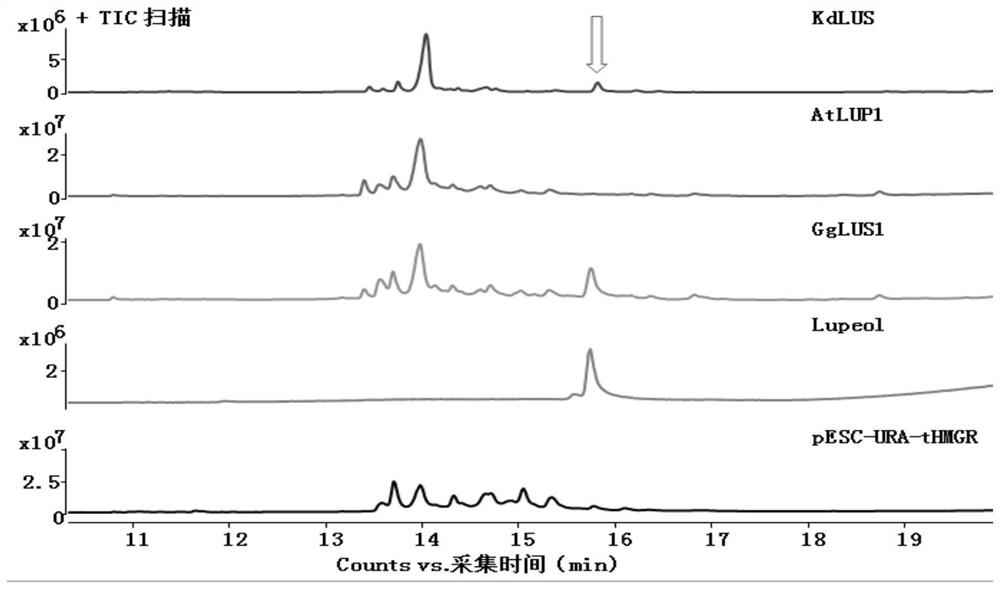
[0081] This embodiment provides a method for synthesizing lupeol, and the specific operation is as follows.
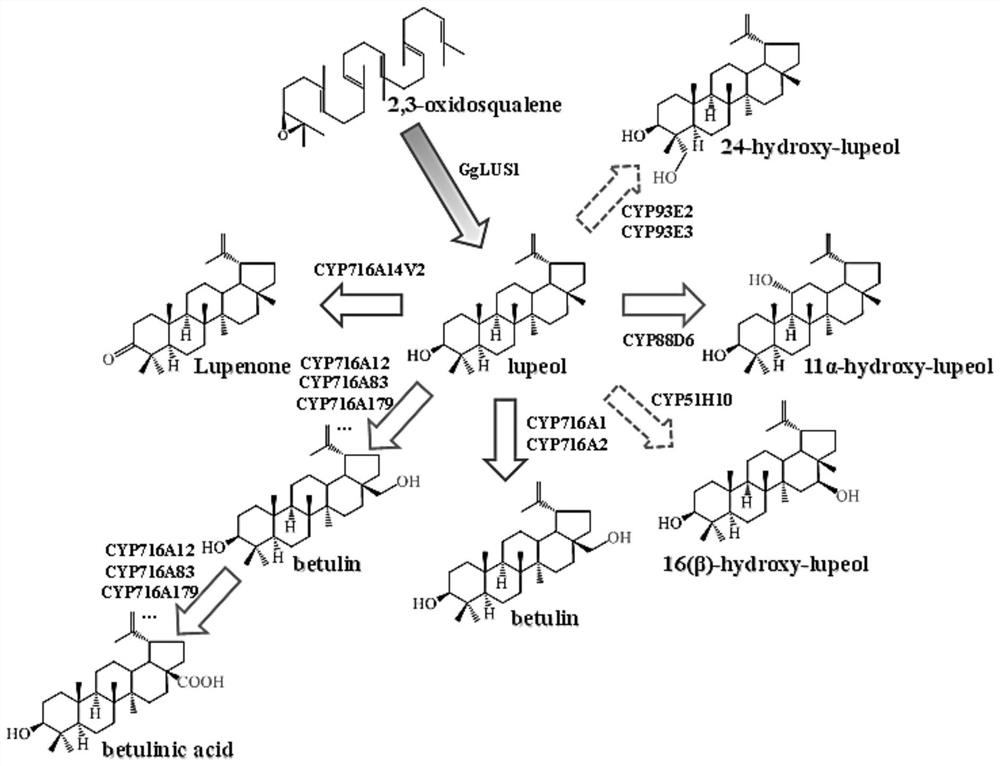
[0082] In this embodiment, the second gene encoding lupeol synthase adopts GgLUS1 gene, the first gene encoding CYP450 enzyme is selected from: CYP51H10, CYP716A83 and CYP88D6; the third gene encoding CPR reductase is selected from: GgCPR1 and AaCPR .
[0083] (1) Acquisition of genes
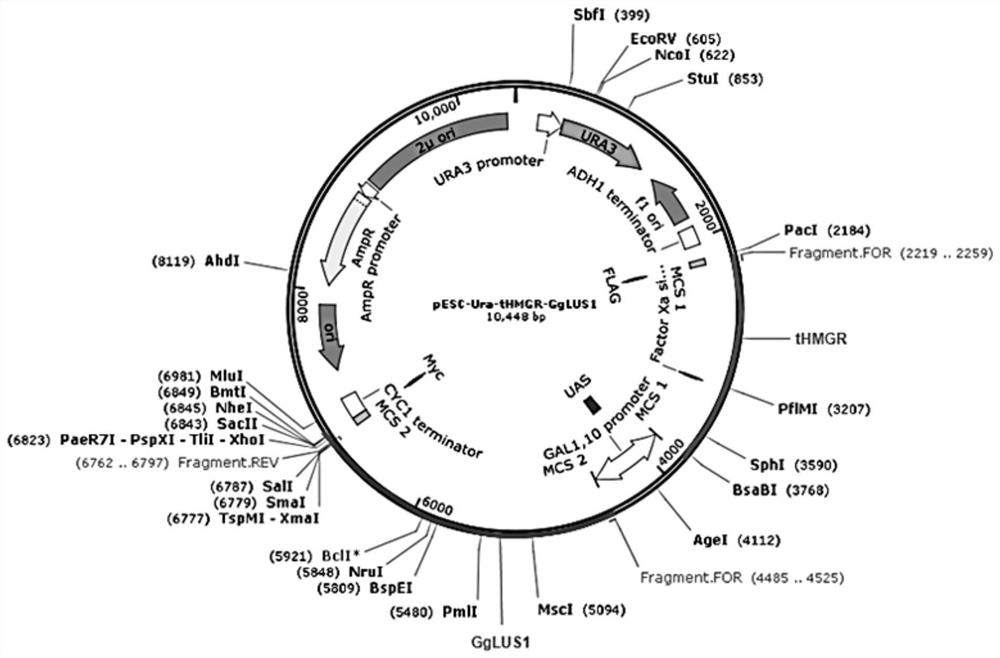
[0084] Look up the nucleotide sequence required for the construction of recombinant organisms on NCBI, find the CDS sequence of the target sequence, and design the primers shown in Table 1. The primer includes a sequence of the candidate gene and a homology arm sequence on the yeast expression vector pESC-tHMGR-Ura or pESC-His or pESC-Leu, the lowercase letters indicate the homologous sequence of the vector, and the uppercase letters indicate the sequence of the gene.
[0085] Table 1 Primer Sequence
[0086]
[0087]
[0088] In Table 1, F is an upstream primer, and R is a dow...
Embodiment 2
[0136] This example provides a method for synthesizing lupeol derivatives. The general process refers to Example 1. The difference is that this example uses the CYP enzyme gene CYP716A83 to catalyze Lupeol. Specific reference Figure 17 In this study, three plasmids containing GgLUS1, CYP716A83 and GgCPR1 were co-transformed into Saccharomyces cerevisiae microbial hosts, and the yeast products after induced expression were isolated and detected. C-28 performed sequential catalytic oxidations and identified the two products of this combination as betulin and betulinic acid.
Embodiment 3
[0138]This example provides a method for synthesizing lupeol derivatives. The general process refers to Example 1. The difference is that this example uses CYP93E3 to catalyze Lupeol. Specific reference Figure 18 In this study, three plasmids containing GgLUS1, CYP93E3 and GgCPR1 were co-transformed into Saccharomyces cerevisiae microbial hosts, and the yeast products after induced expression were isolated and detected. Through the comparison of blank control standards, it was found that CYP93E3 inhibited Lupeol The oxidative modification was identified, and the product of this combination was deduced to be 24-Hydroxy-lupeol based on the known function of CYP93E3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com