Metallographic corrosion method for multiphase austenitic stainless steel weld metal
A technology for austenitic stainless steel and weld metal, applied in the field of metallographic corrosion of multiphase austenitic stainless steel weld metal, can solve the problems of metallographic structure observation, difficulty in simultaneous display and resolution, deterioration of weld metal mechanical properties and Corrosion performance and other issues, to achieve the effect of easy quantitative control, good reproducibility, corrosion conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
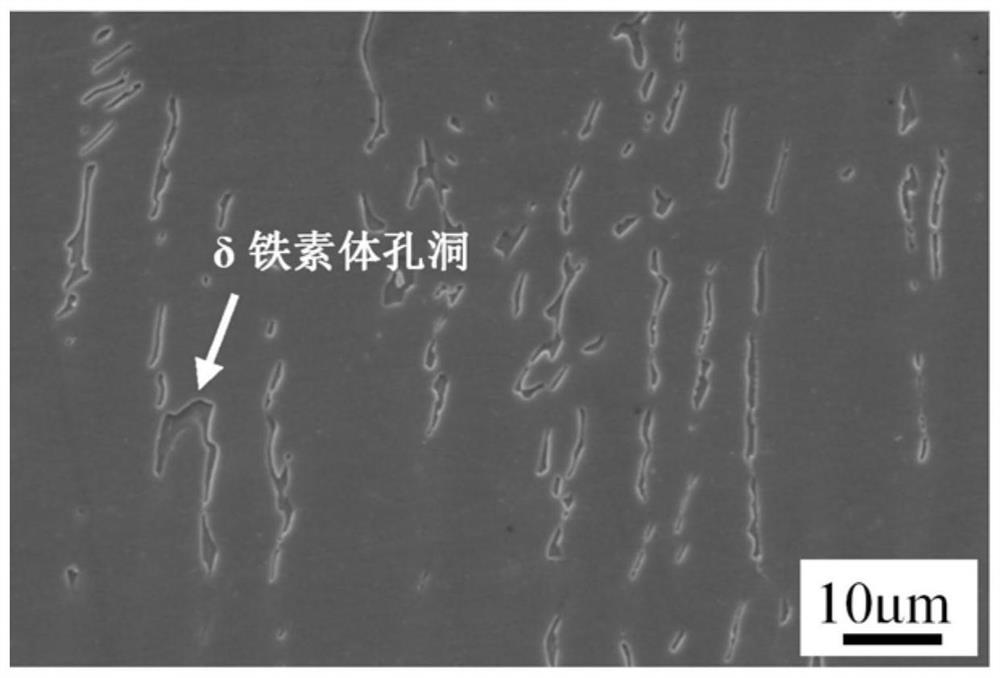
[0031] Prepare the mixed aqueous solution (metallographic corrosion solution) of iron trichloride, hydrochloric acid and nitric acid, specifically: the hydrochloric acid of 40g ferric chloride, 30ml concentration 37wt%, the nitric acid of 20ml concentration 65wt% and 80ml water are mixed and made, Afterwards, soak the 316H weld metal sample in the as-welded state in the metallographic corrosion solution for 50 seconds. After taking it out, rinse the sample with water and alcohol in turn, and dry it with hot air. The metallographic photos are as follows: Image 6 As shown, the delta ferrite did not fall off during the corrosion process, and the delta ferrite in the austenite matrix can be clearly distinguished.
Embodiment 2
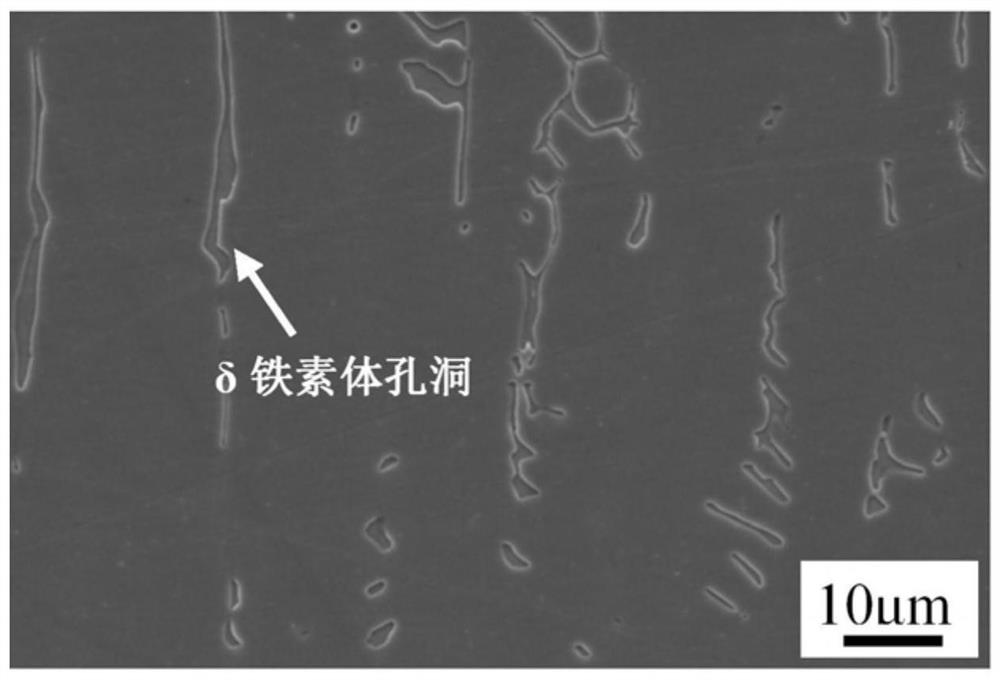
[0033] Prepare an aqueous solution of ferric chloride, hydrochloric acid, and nitric acid. The specific ratio is: 40g ferric chloride + 30ml concentration of 37wt% hydrochloric acid + 20ml concentration of 65wt% nitric acid + 80ml water. Soak the sample in the solution for 40s, after taking it out, rinse the sample with water and alcohol in turn, and dry it with hot air. The metallographic photos are as follows: Figure 7 As shown, under this aging condition, the delta ferrite is partially decomposed into M 23 C 6 ,M 23 C 6 and δ ferrite did not fall off during the corrosion process, and the M in the austenite matrix can be clearly distinguished 23 C 6 and delta ferrite.
Embodiment 3
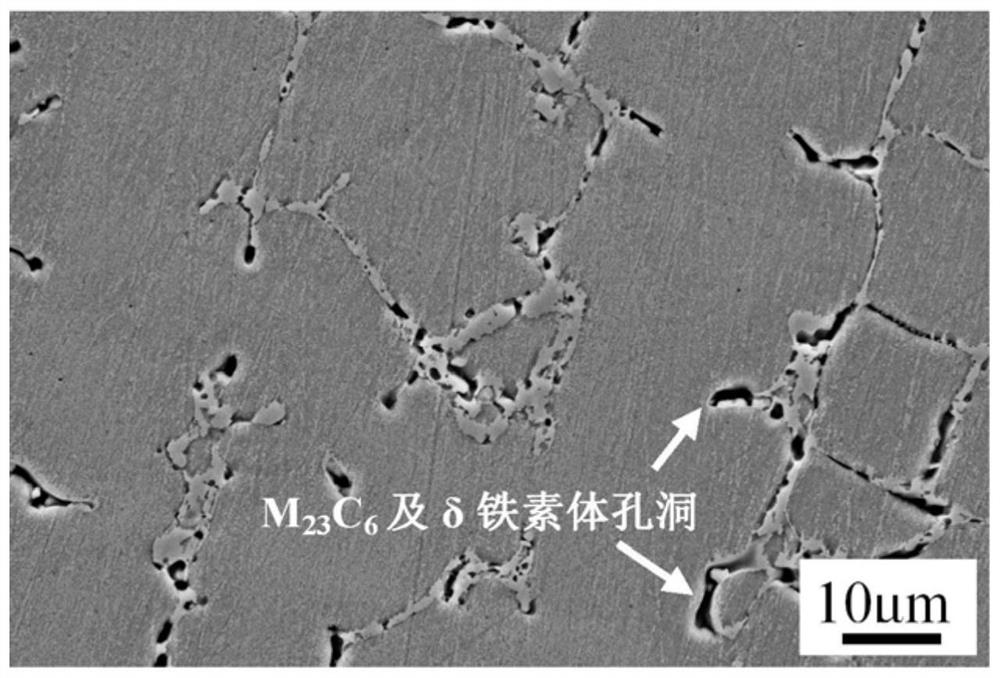
[0035] Prepare ferric chloride, hydrochloric acid, nitric acid aqueous solution, specific ratio is: 40g ferric chloride+30ml concentration 37wt% hydrochloric acid+20ml concentration 65wt% nitric acid+80ml water, after that 750 ℃ / 20h aging state 316H weld metal sample Soak in the solution for 30s, after taking it out, rinse the sample with water and alcohol in turn, dry with hot air, and the metallographic photos are as follows: Figure 8 As shown, under this aging condition, the delta ferrite is completely decomposed into M 23 C 6 and σ phase, M 23 C 6 Both the σ and σ phases did not fall off during the corrosion process, and the δ ferrite transformation products in the austenite matrix can be clearly distinguished.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com