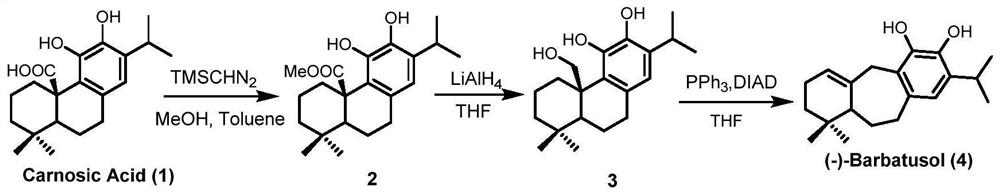
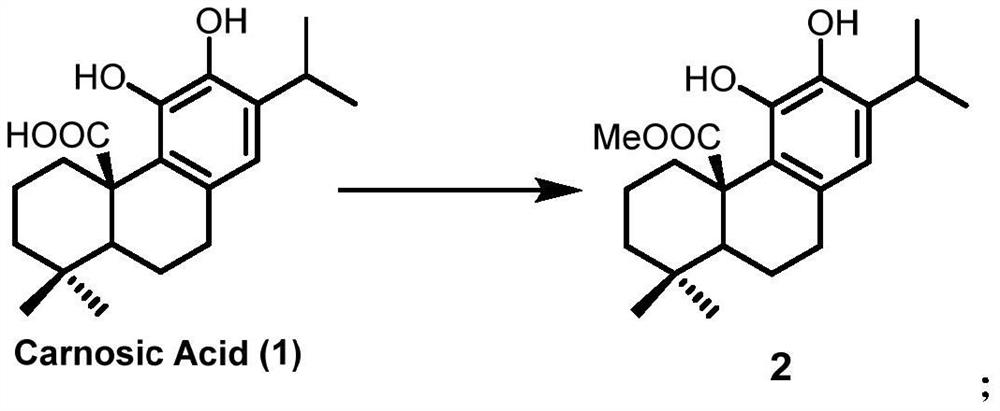
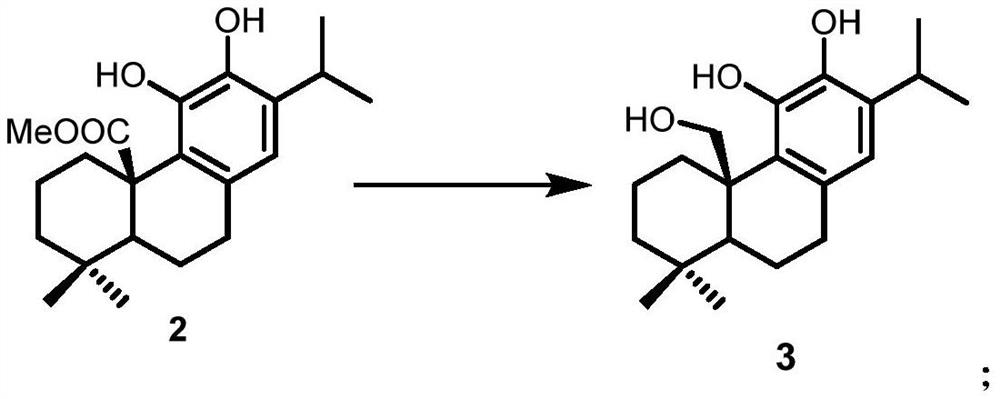
Preparation method of Icetaxane type abietane diterpene
A technology of abietane diterpene and methylation, which is applied in the field of drug synthesis and can solve problems such as poor chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The conception and technical effects of the present invention will be clearly and completely described below in conjunction with the embodiments, so as to fully understand the purpose, features and effects of the present invention. Apparently, the described embodiments are only some of the embodiments of the present invention, rather than all of them. Based on the embodiments of the present invention, other embodiments obtained by those skilled in the art without creative efforts belong to The protection scope of the present invention.
[0047] The chemical reagents used in the following steps were all commercially available starting materials without further processing. The reaction yield of each step is calculated as the separation yield of column chromatography. The reaction of each step is detected by Qingdao silica gel thin layer analysis plate under UV, and the ethanol solution of sulfuric acid and phosphomolybdic acid is infiltrated and heated to develop color. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com