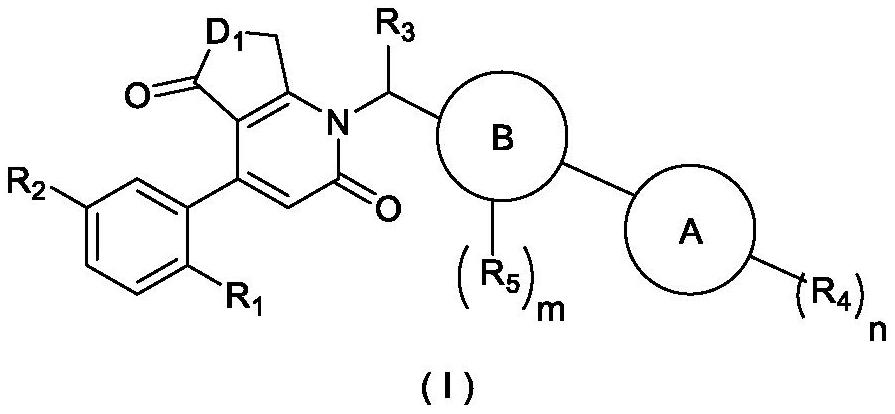
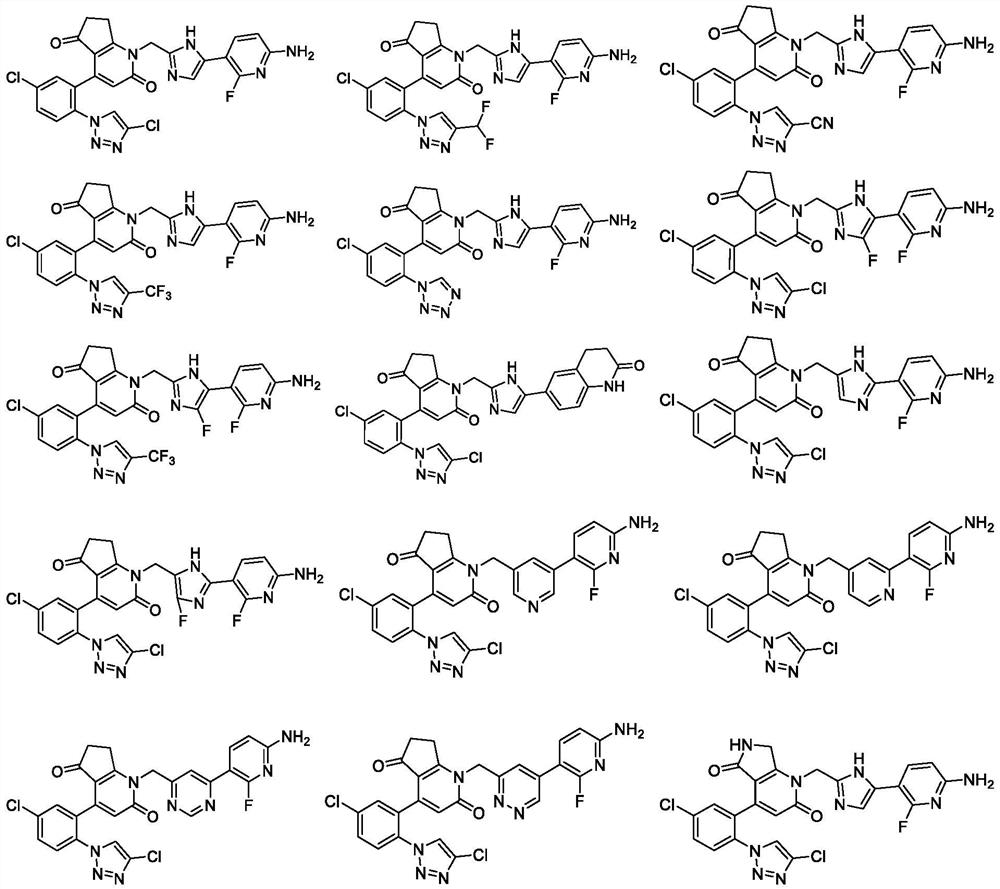
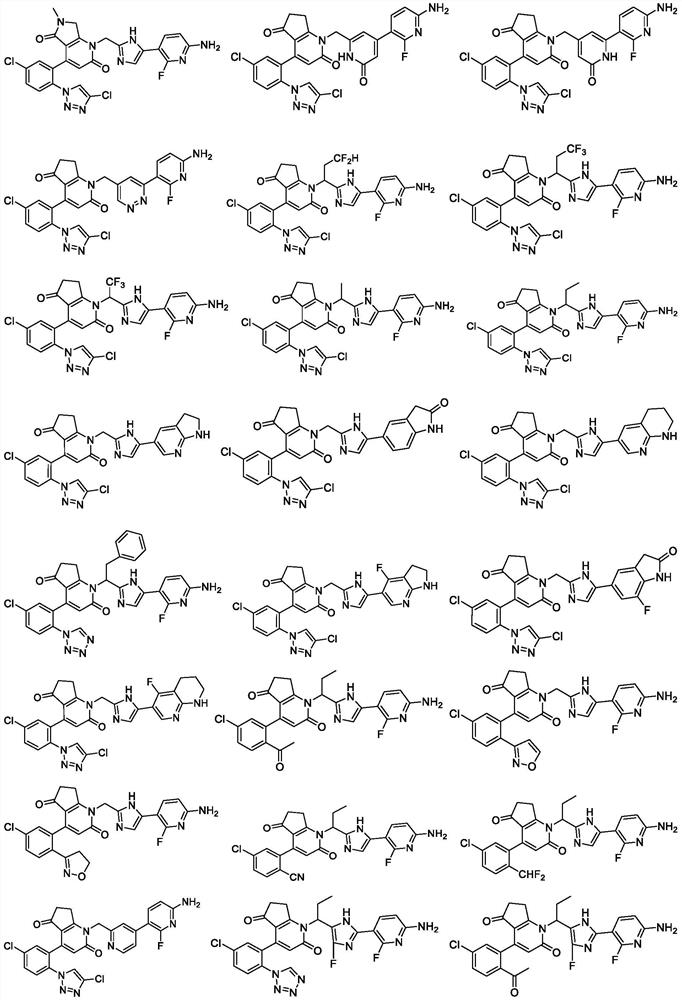
Cyclic keto-pyridone-linked heteroaromatic ring compound as well as preparation method and application thereof
A compound, heteroaryl technology, applied in the field of medicinal chemistry, can solve problems such as unsatisfied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of compound A1
[0092]
[0093] Step 1: Synthesis of Intermediate 1-2
[0094] 1,3 Cyclopentanedione (3.35 g, 34.15 mmol), tert-butyl glycine (2 g, 6.63 mmol) and PTSA (649 mg, 3.42 mmol) were added to toluene (100 mL). Reaction system uses N 2 Under protection, the temperature was raised to 130° C., and the reaction was carried out for 3 hours. The reaction solution was spin-dried, dissolved in 500 mL of EtOAc, and washed with saturated NaHCO 3 Washed with aqueous solution, dried, filtered, and spin-dried to obtain 5 g of the target product 1-2 as a yellow solid, yield: 69%.
[0095] Step 2: Synthesis of Intermediates 1-3
[0096]Compound 1-2 (1.7 g, 8.05 mmol) and bis(2,4,6-trichlorophenyl)malonate (3.95 g, 8.05 mmol) were added to toluene (100 mL). The reaction solution was stirred under microwave at 120°C for 2h. The reaction solution was cooled to room temperature and spin-dried to obtain a crude product, which was purified b...
Embodiment 2
[0121] Embodiment 2: the preparation of compound A2
[0122]
[0123] Wherein intermediate 2-2 is synthesized as follows.
[0124]
[0125] Compound 2-1 (domestic) (1.0g, 6.80mmol) was dissolved in 10mL DCE, at room temperature, bromoacetyl bromide (2.0g, 10.20mmol) and aluminum chloride (6g, 44.88mmol) were added, and the reaction After the solution was stirred overnight at 45°C, the reaction solution was poured into ice water, and a large amount of solids precipitated out. After the solids were filtered, the solids were washed with water and ethyl acetate. After the solids were dried, ethyl acetate was added for recrystallization and purification to obtain the intermediate 2-2, white solid, 350 mg, yield 20%. LC-MS: m / z 270.0 (M+H) + , Purity: 89% (UV254).
[0126] Example A2 was obtained from intermediates 1-10 and 2-2 by a similar synthesis procedure to A1. LC-MS: m / z 586.2 (M+H) + . 1 HNMR (400MHz, DMSO-d 6 )δ12.17(s,1H),10.05(s,1H),8.64(s,1H),7.78(dd,J=8.6,2...
Embodiment 3
[0127] Embodiment 3: the preparation of compound A3
[0128]
[0129] Wherein intermediate 3-5 is synthesized as follows:
[0130]
[0131] Step 1. Synthesis of Intermediate 3-1
[0132] Add intermediate 1-6 (1150mg, 4.98mmol), 3,3-diethoxyprop-1-yne (956mg, 7.47mmol) and toluene (10mL) into a 100mL round bottom flask, heat up to 110°C, stir Reaction 16h. LC-MS showed product formation and the reaction was complete. The reaction solution was spin-dried and purified by normal phase column (PE / EA=0-50%) to obtain 1.40 g of intermediate 3-1 as yellow oil, yield: 78%. LCMS:m / z362.0(M+H) + .
[0133] Step 2. Synthesis of Intermediate 3-2
[0134] Add HCl (20 mL) and dioxane (20 mL) into a 100 mL round bottom flask, then add compound 3-1 (1.2 g, 3.34 mmol) into the above mixed solution, heat up to 30° C., and react for 16 h. LC-MS detection showed that the product was generated, and the reaction ended. The reaction solution was diluted with water (40 mL), and extracted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com