Recombinant rabies virus of chimeric canine parvovirus VP2 gene and application of recombinant rabies virus
A technology of rabies virus and canine parvovirus, applied in the field of immunology, can solve the problems of high production cost and short duration of antibody response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention provides the preparation method of the recombinant rabies virus, comprising the following steps: 1) using the canine parvovirus genome as a template, PCR amplification obtains the VP2 gene; 2) connecting the VP2 gene to the pBNSP plasmid to obtain the recombinant plasmid pBNSP -CPV-VP2; the pBNSP plasmid carries the full-length cDNA of SAD-B19 strain; 3) the recombinant plasmid pBNSP-CPV-VP2, helper plasmid N, helper plasmid P, helper plasmid L, helper plasmid G and helper plasmid The recombinant rabies virus was obtained after pCAGGS-T7 was mixed and transfected into cells.
[0038] In the present invention, the VP2 gene is obtained by PCR amplification using the canine parvovirus genome as a template. In the present invention, the source of the canine parvovirus genome is not particularly limited, and the canine parvovirus genome from a conventional source in the field can be used. In the specific implementation process of the present invention, the cani...
Embodiment 1
[0055] 1. Centrifuge the clinically collected suspected CPV sample (number CPV-LZ-1) at 5000rpm for 10min, take the supernatant, and then follow the Tiangen Feces Genomic DNA Extraction Kit
[0056] According to the instruction manual, the CPV genomic DNA was extracted and then amplified by PCR.
[0057] The primer sequences are listed in Table 1.
[0058] Table 1 CPV VP2 gene amplification primers
[0059]
[0060] (2) Utilize primer CPV1 / CPV2, carry out PCR amplification according to the system shown in Table 2:
[0061] Table 2 PCR amplification system
[0062]
[0063] Amplification program: 95°C for 5min; 94°C for 30s, 56°C for 30s, 72°C for 2min, 30 cycles; 72°C for 10min. The amplified PCR product was electrophoresed on 1% agarose gel.
[0064] The amplified product was cloned into the pMD19-T Simple vector (purchased from Dalian TaKaRa Company), the ligated product was transformed into JM109 competent cells (purchased from Dalian TaKaRa Company), the plasmid ...
Embodiment 2
[0066] Cloning of VP2 gene of CPV strain into pBNSP
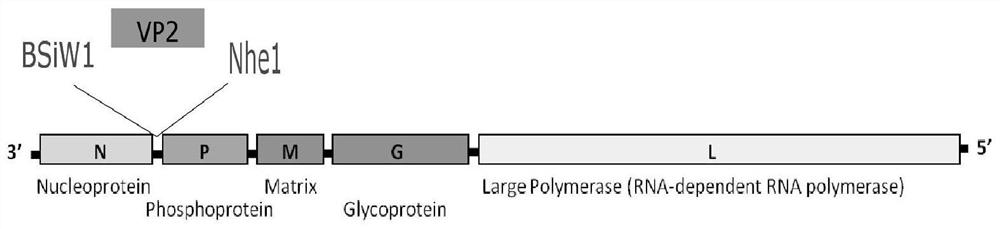
[0067] 1. For the strategy of constructing the recombinant plasmid pBNSP-CPV-VP2, see figure 1 shown.
[0068] (1) utilize primer CPV-VP2 / F and CPV-VP2 / R (see Table 1) to amplify the VP2 gene (sequence shown in SEQ ID No.1) of the CPV strain selected in the embodiment 1, CPV-VP2 The expected amplification length of / F and CPV-VP2 / R primers is 1755bp (the amplification reaction system is shown in Table 2).
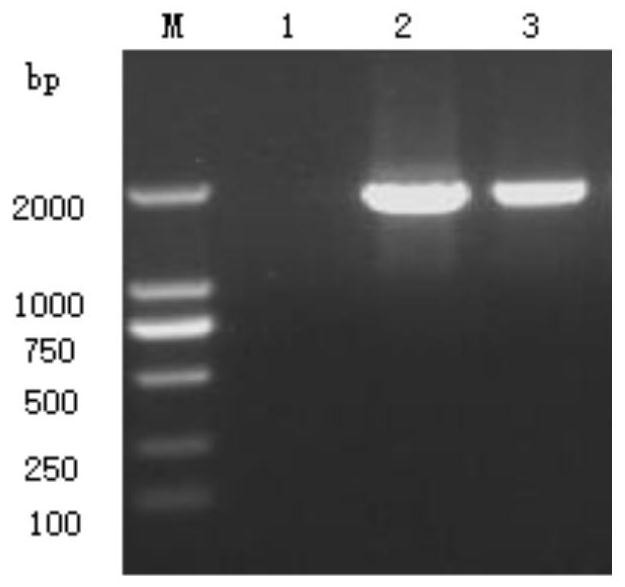
[0069] The PCR amplification program was: 95°C for 5 minutes; 94°C for 30s, 56°C for 30s, 72°C for 2min, 30 cycles; 72°C for 10min.
[0070] (2) After the reaction, 5 μL of the PCR product was taken for electrophoresis detection on 1.0% agarose gel. results as seen figure 2 As shown, a specific DNA electrophoresis band with a size of 1755bp was obtained, which was consistent with the experimental expectation.
[0071] (3) The VP2 gene amplified by PCR is recovered and purified by gel cutting, and the purified VP2 g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com