GPR120 protein receptor inhibitor as well as preparation and application thereof
A C1-C6, C1-C4 technology, applied in the field of small molecule protein inhibitors, can solve problems such as unobserved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
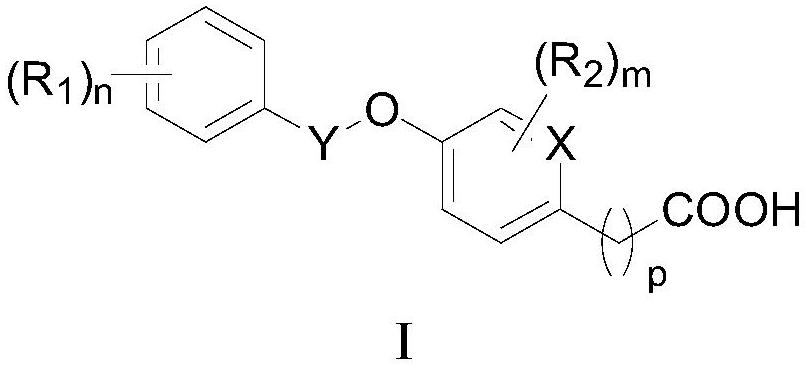
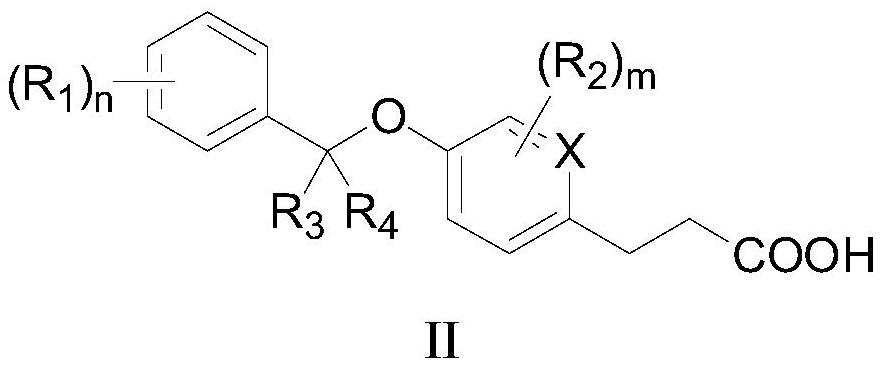
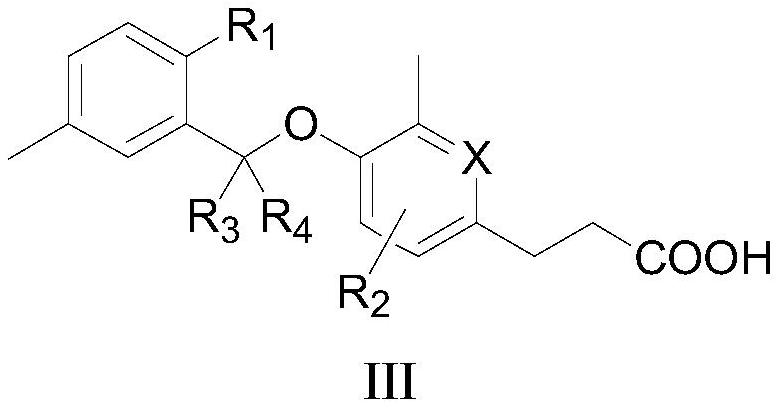
[0072] The preparation of formula I compound
[0073] The present invention also provides a method for preparing the compound as described in the first aspect of the present invention, comprising the steps of:
[0074]
[0075] In an appropriate solvent, react with alcohol Ia and phenol Ib to obtain compound Ic, and obtain a compound of formula I after deprotection, wherein LG is a leaving group, preferably C 1 -C 4 alkyl.
[0076] Pharmaceutical compositions and methods of administration
[0077] Since the compound of the present invention has excellent GPR120 inhibitory activity, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, and compounds containing the compound of the present invention as the main active ingredient The pharmaceutical composition can be used to prevent and / or treat diseases related to GPR120 signaling pathway (for example, cancer).
[0078] The pharmac...
Embodiment 1
[0092] 3-(4-((2-isopropyl-5-methylbenzyl)oxy)-3-methylphenyl)propanoic acid
[0093]
[0094] Step 1: Synthesis of (2-isopropyl-5-methylphenyl)methanol
[0095] A solution of 2-isopropyl-5-methylbenzaldehyde (100 mg, 0.62 mol) in THF (5 mL) was cooled to -78 °C. LAH (1.0 M in THF, 0.8 mL, 0.8 mmol) was added at this temperature and the reaction was allowed to warm to room temperature. The reaction was poured into ice-water mixture, and extracted with ethyl acetate (20 mL*2). The combined organic layers were washed with brine (10 mL), dried over sodium sulfate, filtered and concentrated. The residue was purified by column chromatography to afford the title compound as a yellow oil (80 mg, 80% yield). MS(ESI): m / z 165.5(M+H) + .
[0096] Step 2: Synthesis of methyl 3-(4-(((2-isopropyl-5-methylbenzyl)oxy)-3-methylphenyl)propanoate
[0097] (2-Isopropyl-5-methylphenyl)methanol (80mg, 0.49mmol), methyl 3-(4-hydroxy-3-methylphenyl)propionate (105mg, 0.54mmol) and PPh 3 (14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com