Method for preparing dapagliflozin intermediate by one-pot method
A technology for an intermediate, chlorobenzoic acid, applied in the field of one-pot preparation of dapagliflozin intermediates, can solve problems such as complicated steps and environmental pollution, and achieve the effects of improving product purity, simple post-processing operations, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
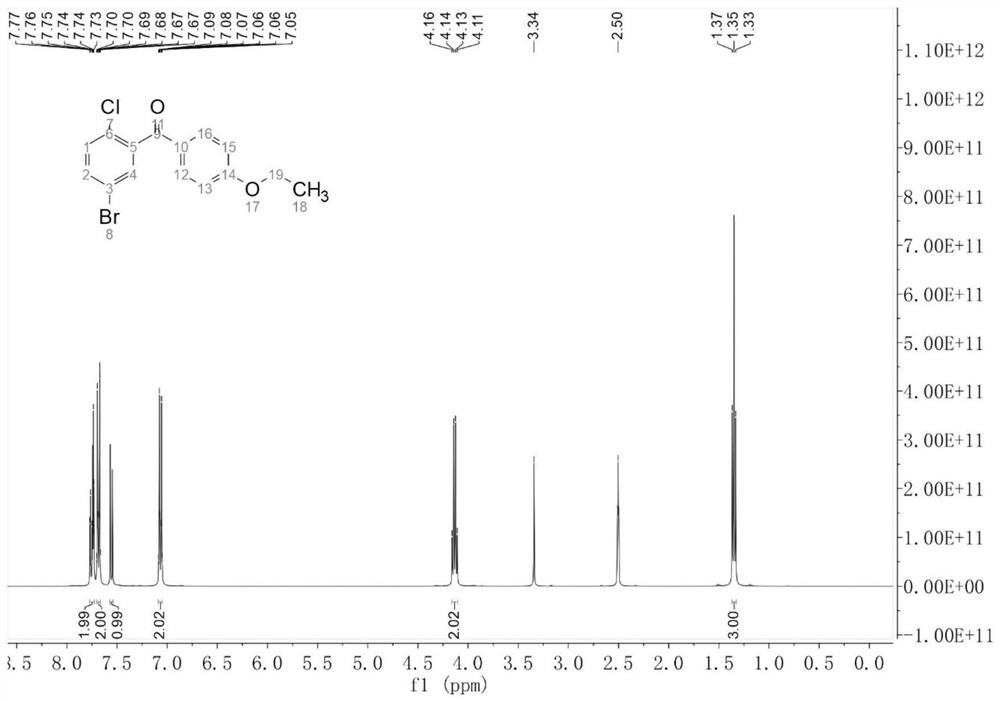
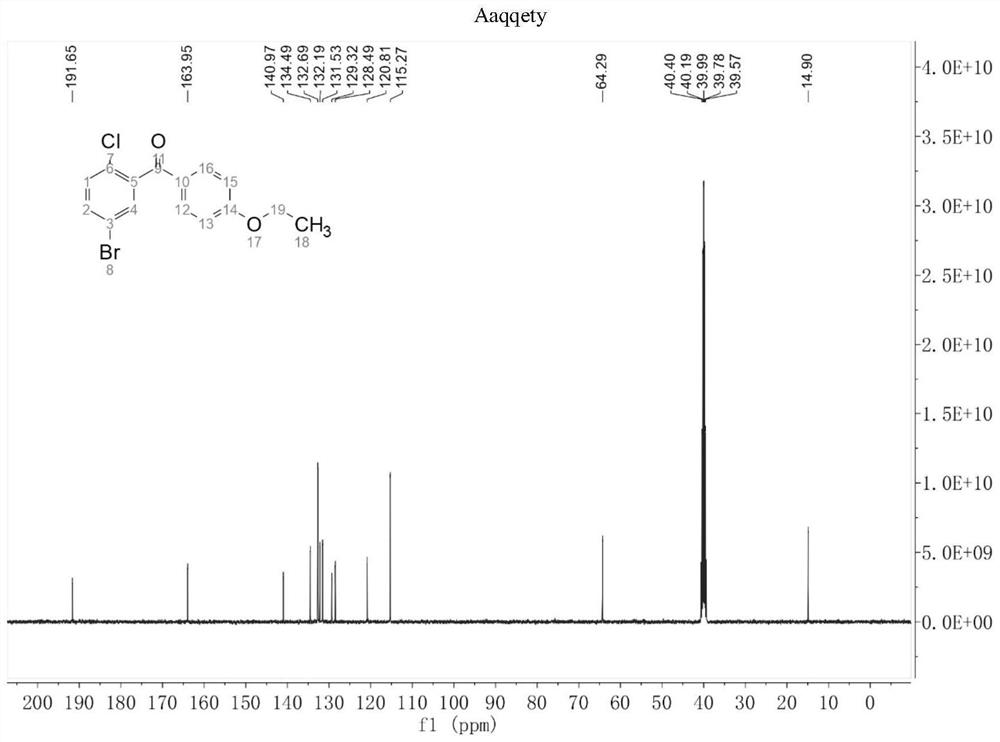
[0034] Add 20 mL of 1,4-dioxane, 5-bromo-2-chlorobenzoic acid (2.35 g), red phosphorus (123.89 mg), sodium bromide (51.44 mg), anhydrous sodium carbonate powder ( 105.99mg), α-Fe 2 o 3 (128.00mg), potassium persulfate (5.40g) and 4A type molecular sieve (2.00g), after stirring evenly at 40°C, after adding phenetole (1.83g) dropwise at 0.184mL per minute, react at 40°C After 4 hours, after quenching, layering, drying and concentrating, recrystallization was carried out by absolute ethanol to obtain a white powdery solid, i.e. (5-bromo-2-chlorophenyl) (4-ethoxyphenyl) ketone ( 3.04g, the yield is 90%, and the purity monitored by HPLC is 99.5%, and its hydrogen spectrum and carbon spectrum results are as figure 1 with figure 2 shown.
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ7.77–7.73(m,2H),7.70–7.67(m,2H),7.56(d,J=8.4Hz,1H),7.09–7.05(m,2H),4.13(q,J=7.0Hz ,2H),1.35(t,J=7.0Hz,3H);
[0036] 13 C NMR (101MHz, DMSO-d 6 )δ191.65, 163.95, 140.97, 134.49, 132.69, 132.19, 131.53, 1...
Embodiment 2
[0038] Add 20 mL of 1,4-dioxane, 5-bromo-2-chlorobenzoic acid (2.35 g), red phosphorus (123.89 mg), sodium bromide (51.44 mg), anhydrous sodium carbonate powder ( 105.99mg), α-Fe 2 o 3 (128.00mg), potassium persulfate (5.40g) and A4 molecular sieve (2.00g), after stirring evenly at 10°C, after adding phenetole (1.83g) dropwise at 0.184mL per minute, react at 10°C for 5 After 1 hour, quench, separate layers, dry and concentrate, and then recrystallize from absolute ethanol to obtain a white powdery solid, which is detected as (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone (2.63 g, 72% yield, 99.0% purity by HPLC).
Embodiment 3
[0040] Add 20 mL of 1,4-dioxane, 5-bromo-2-chlorobenzoic acid (2.35 g), red phosphorus (123.89 mg), sodium bromide (51.44 mg), anhydrous sodium carbonate powder ( 105.99mg), α-Fe 2 o 3(128.00mg), potassium persulfate (5.40g) and A4 molecular sieve (2.00g), after stirring evenly at 60°C, after adding phenetole (1.83g) dropwise at 0.184mL per minute, react at 60°C for 3 Hours later, after quenching, layering, drying and concentrating, recrystallization by absolute ethanol gave a white powdery solid, i.e. (5-bromo-2-chlorophenyl) (4-ethoxyphenyl) ketone (2.70 g, the yield is 80%, and the yield monitored by HPLC is 99.0%).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap