Improved methods for inducing tissue regeneration and senolysis in mammalian cells
A technology of mammals and cells, which is applied in the direction of animal cells, vertebrate cells, biochemical equipment and methods, and can solve problems such as limited methods of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
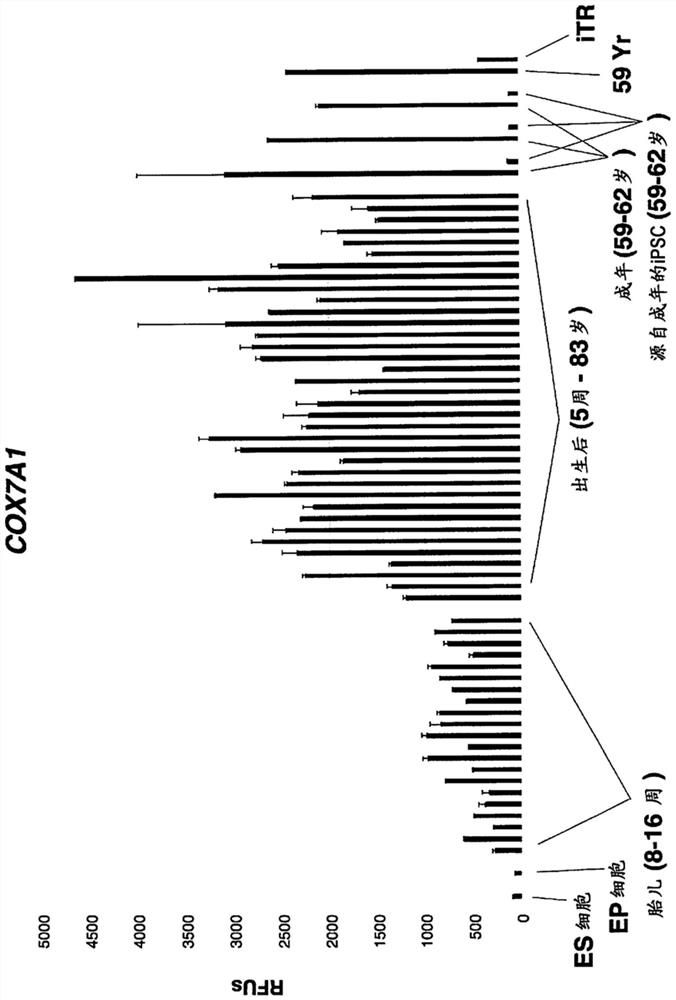
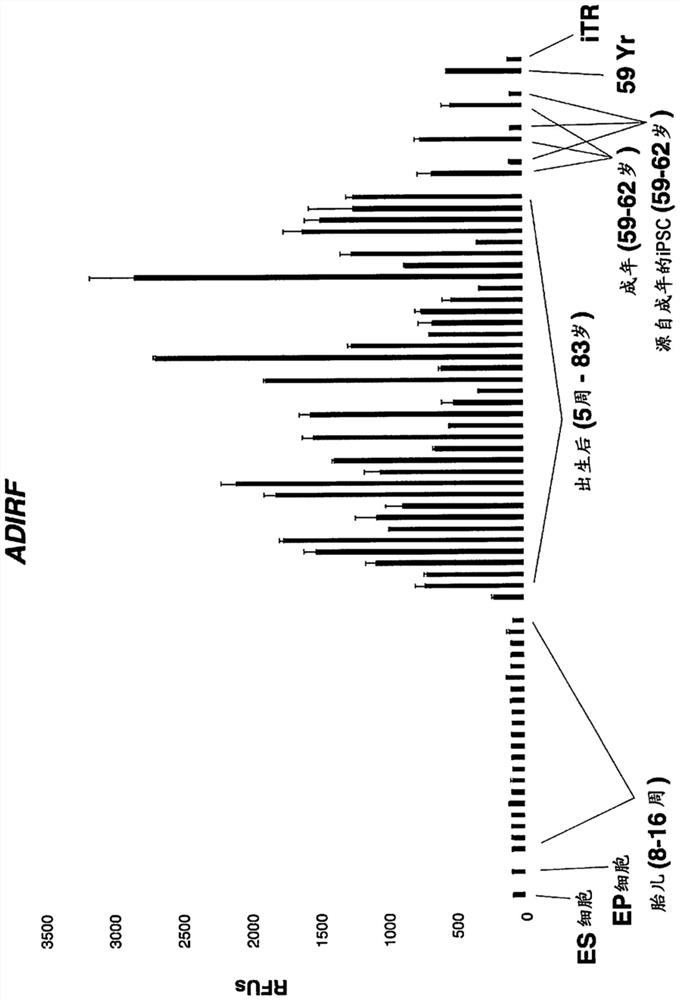
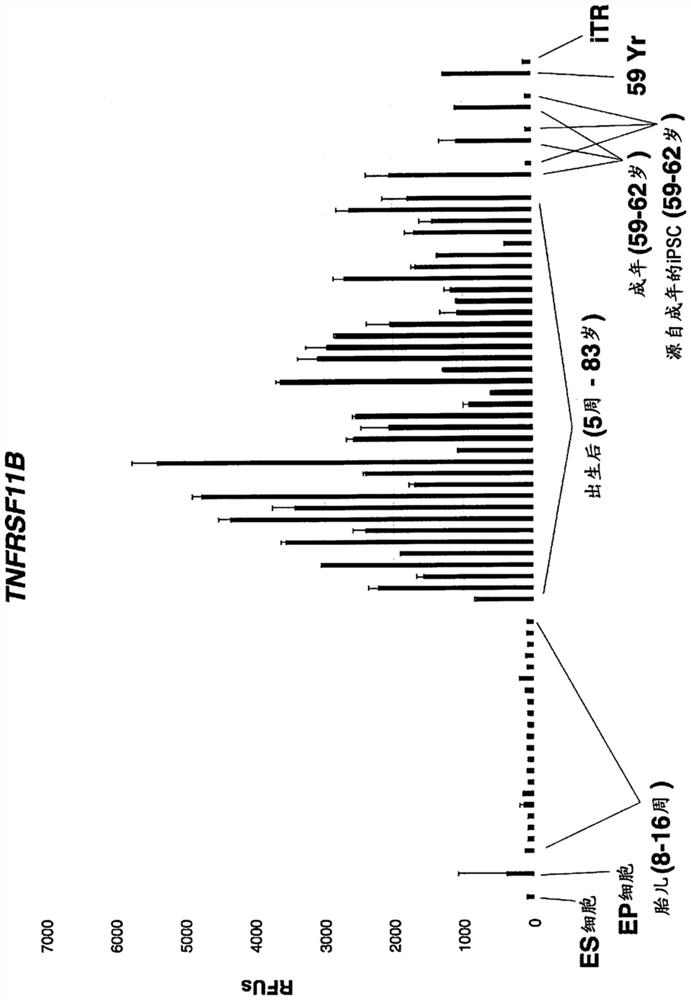
[0215] Example 1. Human adult skin fibroblasts were subjected to a low-throughput screen for iTR factors using PCR to measure the reduction of COX7A1 expression as a marker for iTR.
[0216] Approximately 200 combinations of candidate iTR factors described herein were screened under low-throughput conditions as a first assay, COX7A1 expression was measured by qPCR. Conditions showing a marked reduction in COX7A1 expression were used to prepare RNA for Illumina bead array- or RNA-sequencing-based transcriptomic analysis. Optimal conditions leading to the greatest reduction in COX7A1 levels while maintaining viable cells were 0.5 mM valproic acid, 10 uM CHIR99021, 10 uM RepSox, 10 uM Tranylpromine (Parnate), 10 uM tranylpromine (Parnate), 5OuM Forskolin, 5uM TTNPB, and then 10OnM 3-Deazaneplanocin A (DZNep) were added to the above mixture for another 14 days, then only 1.OuM PD0325901 and 1OuM CHIR99021 were used for the last 7 days. All factors were used in cell growth mediu...
Embodiment 2
[0221] Example 2. Screening for iTR factors capable of inducing lysis of senescent cells in senescent cells or CSCs.
[0222] Optimal conditions were determined to distinguish the expected increase in apoptosis from cells with prefetal gene expression patterns. Mesenchymal (4D20.8 and MSC, respectively) and vascular endothelial cells (30MV2-6 and HAEC, respectively) of embryonic and adult origin were stimulated with different apoptotic stimuli including two concentrations of camptothecin (CPT) 、H 2 o 2 And thapsigargin (TG) and vehicle control. Compounds were administered 24 hours prior to analysis. After treatment, apoptosis was monitored by TUNEL staining using the In Situ Cell Death Detection kit (Roche, Cat# 12156792910) following the manufacturer's instructions. like Figure 7 As shown, thapsigargin at 3.7 nM provided a statistically significant increase in apoptosis in both embryonic mesenchymal and endothelial cells compared to their adult counterparts. Therefor...
Embodiment 3
[0223] Example 3. Method for making an EGFP reporter of COX7A1 expression for use in high-throughput iTR screening.
[0224] Insertion of IRES-EGFP downstream of the COX7A1 locus in TERT-immortalized human foreskin fibroblasts. To achieve this, two candidate guide RNAs (gRNAs) were designed and cloned to target the COX7A1 locus. gRNA-mediated CRISPR / Cas9 modification leads to targeted double-strand breaks (DSBs). A donor plasmid carrying an IRES-EGFP cassette was constructed based on the position of an active gRNA to serve as a DNA repair template. Co-transfection of gRNA and donor plasmids into TERT-immortalized human foreskin fibroblasts mediates homology-directed repair (HDR), thereby enabling IRES-EGFP to be knocked into the target region of COX7A1 ( Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com