Flavonol glycoside derivatives and application and preparation method thereof
A technology of flavonol glycoside derivatives and drugs, which is applied in the field of medicine and can solve problems such as poor response, adverse reactions, and slow curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Separation of Main Components in Long Stem Golden Waist
[0039] The present embodiment is the separation method of the main components in the long stem golden waist, including:
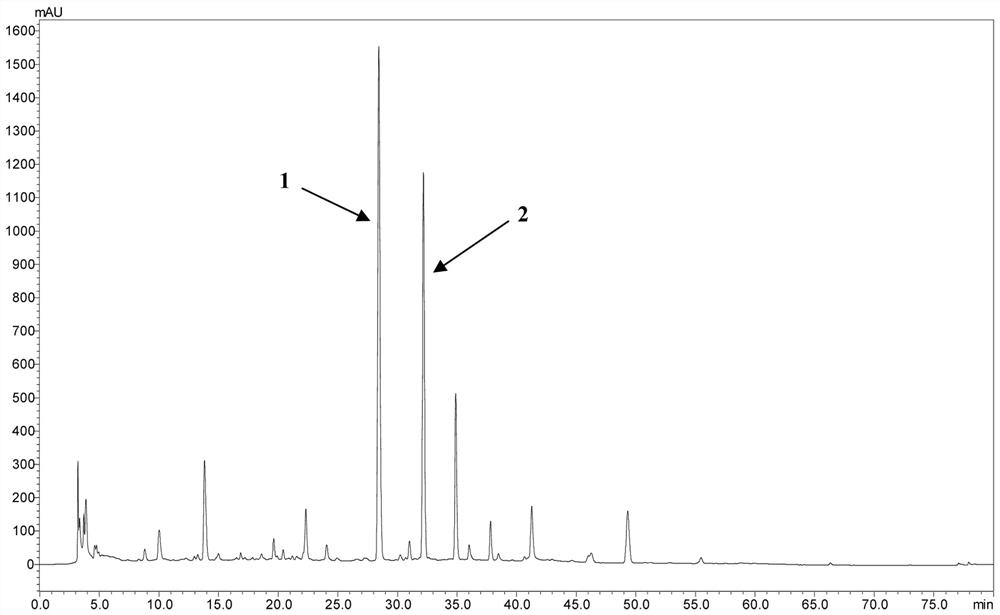
[0040] (1) Take 6.0 kg of dried whole herb of Chrysanthemum long stems, crush it properly, put it in a percolation barrel, add 70% ethanol for percolation extraction, combine the extracts, concentrate under reduced pressure to recover the solvent, and obtain 2.5 kg of extract. A small amount of extract was dissolved in 70% methanol, passed through a 0.45 μM filter membrane, and 10 μL of the resulting filtrate was injected into a high-performance liquid chromatography (HPLC) to obtain the HPLC fingerprint of the 70% alcohol extract of Aurantia chinensis ( image 3 ). Such as figure 1 , figure 2 , image 3 As shown, its main components are compound 1 and compound 2.
[0041] (2) Take 2.4 kg of extract, heat and knead with 500 mL of 10% ethanol to dissolve, then add 500 mL of dist...
Embodiment 2
[0046] Example 2 This example is the structural identification of the above-mentioned compound 1 and compound 2, including two parts:
[0047] (1) Structural identification of compound 1
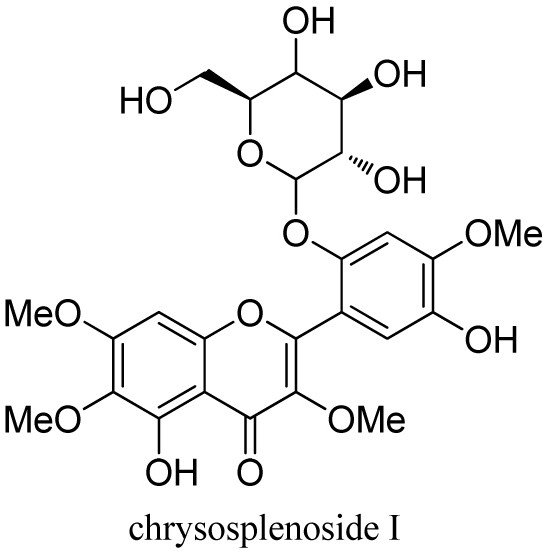
[0048] Compound 1 is a yellow-brown amorphous powder, which is easily soluble in methanol. HR-ESI-MS gives quasi-molecular ion peaks m / z : 553.1545([M+H] + , C 25 h 29 o 14 , the theoretical calculation value is 553.1552), and its molecular formula is determined to be C 25 h 28 o 14 .
[0049] The nuclear magnetic resonance (NMR) hydrogen spectrum of compound 1 ( 1 H) [such as figure 2 Shown] and carbon spectrum ( 13 C) [such as image 3 The data shown are very similar to that of a known pentamethoxyflavonol glycoside, chrysosplenoside H. with chrysosplenoside H 1 Compared with H NMR spectrum, compound 1 has one more phenolic hydroxyl proton signal in the low field region δ H 9.00 (1 H, brs), and there is one less methoxy signal in the high field region δ H 3.93 (3 H...
Embodiment 3
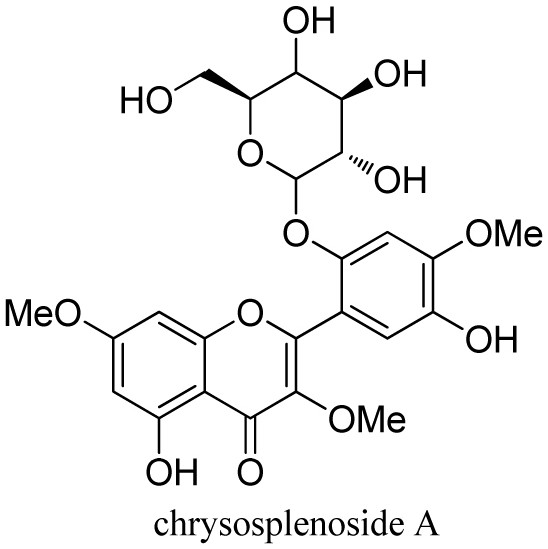
[0059] This example is about the acute toxicity evaluation of chrysosplenoside A and chrysosplenoside I. The evaluation method is: in accordance with the "Technical Guidelines for Acute Toxicity Test of Chemical Drugs" issued by China Food and Drug Administration (CFDA) and acute toxicity classification According to the guiding principles of the method, 2 g / kg was used as the initial dosage of chrysosplenoside I and chrysosplenoside A, and the mice were orally administered once, and the acute toxicity test in mice was carried out.
[0060] The animal grouping and dosing plan are as follows: randomly select 40 mice, half male and half male, and mark them with 3% picric acid solution; the experiment is divided into 4 groups, with 5 males and 5 females in each group, which are blank control group and vehicle control group respectively. group, chrysosplenoside I administration group, and chrysosplenoside A administration group; the test drug was ultrasonically dissolved with 0.5% C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com