Flavonol glycoside derivatives and their use and preparation method
A technology for converting flavonol glycoside derivatives and ethanol, applied in the field of medicine, can solve the problems of long treatment process, severe IC cannot be used for a long time, slow curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Separation of the main ingredients in the gold waist
[0039] This embodiment is the separation method of the main components in the diaphragm gold waist, including:
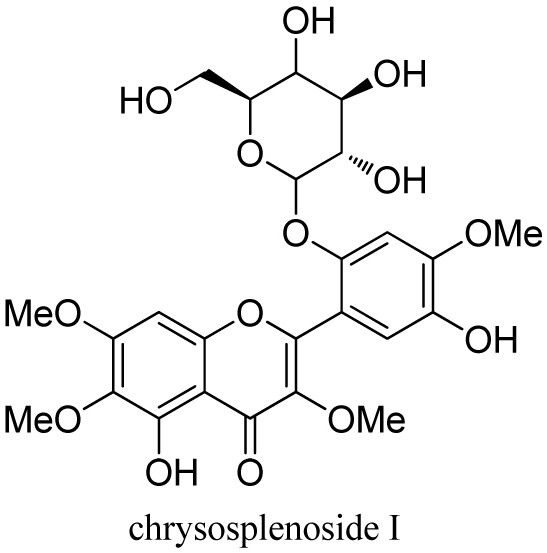
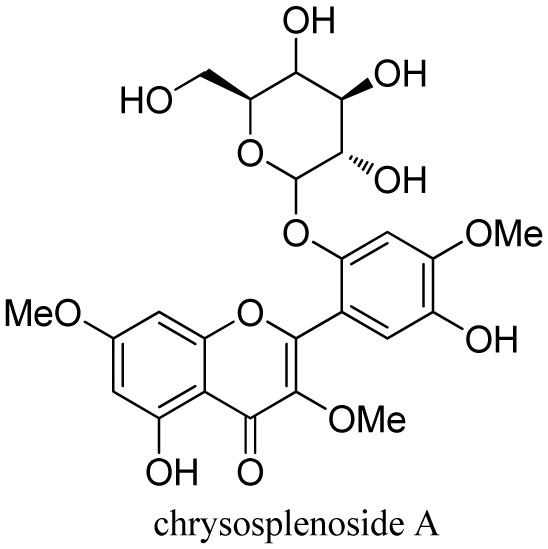
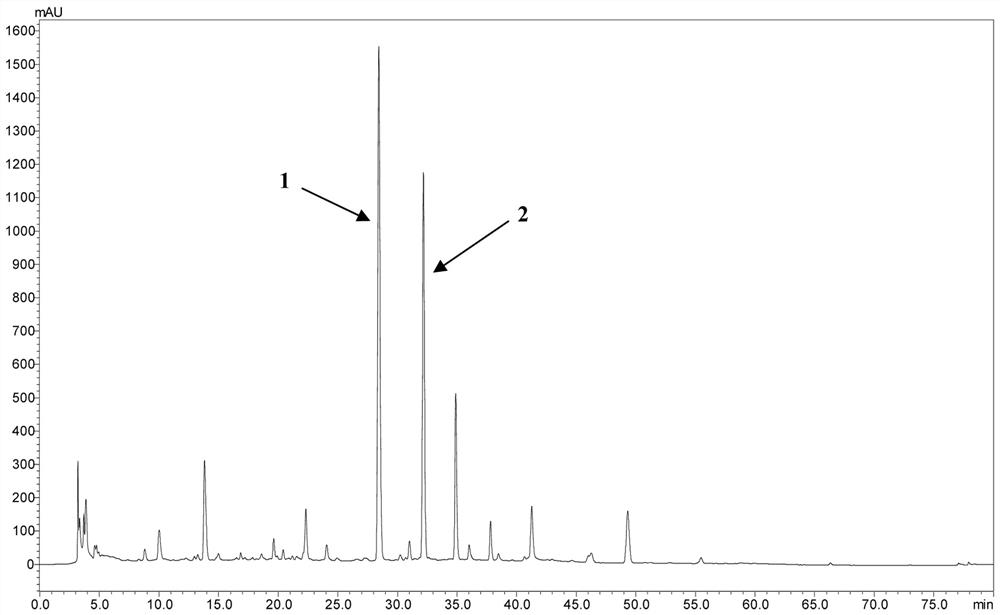
[0040] (1) Drying the gold waist and dry the whole steroid. The whole grass is 6.0 kg, properly pulverized, placed in a permeable barrel, add 70% ethanol osmication extraction, combined extract, concentrate the solvent, and soon it. 2.5 kg. Take a small amount of dipper, dissolved in 70% methanol, a 0.45 μm filter, and the resulting filtrate was 10 μl of injection of high-performance liquid chromatograph (HPLC), and the HPLC fingerprint of 70% alcohol of the long stem gold waist ( image 3 ). like figure 1 , figure 2 , image 3 As shown, its main component is Compound 1 and Compound 2.
[0041] (2) Take 2.4 kg of dipping and heat it with 500 ml 10% ethanol, then add 500 mL of distilled water to disperse.
[0042](3) Dispersion liquid HP-20 macroporous adsorption resin column, with distilled water, 10%...
Embodiment 2
[0046] EXAMPLE 2 This example is a structural identification of the compound 1 and Compound 2, including two parts:
[0047] (1) Structure identification of Compound 1
[0048] Compound 1 is a yellow-brown non-forming powder, which is soluble in methanol. HR-ESI-MS gives the alignment molecular ion peak m / z : 553.1545 ([M + H] + , C 25 Hide 29 O 14 The theoretical calculation value is 553.1552), determine its molecular formula C 25 Hide 28 O 14 .
[0049] Nuclear magnetic resonance (NMR) hydrogen spectrum of Compound 1 1 H) figure 2 Distant] and carbon spectrum ( 13 C) image 3 The data shown is very similar to a known fiven oxagleanoside h. With Chrysosplenoside H 1 Compound 1 is more phenolic hydroxyprom signal in the low field region than the H NMR spectrum. δ H 9.00 (1 H, BRS), while a signal of a methoxy group in the high field area δ H 3.93 (3 h, s); with Chrysosplenoside H 13 C NMR spectrum compared to a signal of 1 methoxy group δ C 56.9. This suggests that Compound 1 is ...
Embodiment 3
[0059] This example is about Chrysosplenoside A and Chrysosplenoside I acute toxicity evaluation, the evaluation method is: "Chemical Drug Acute Test Technical Guidelines" and acute toxicity in accordance with China Food and Drug Administration, CFDA The guidelines of the law were carried out as the initial administration of Chrysospleniside I and Chrysosplenoside A, and mice were performed in a mouse acute toxicity test.
[0060] The animal grouping and administration protocol was as follows: randomly selected 40 mice, more than half of female, and labeled 3% bitter acid solution; the experiment was divided into 4 groups, each group of male, female each, respectively, blank control group, mediating control Group, Chrysosplenoside I administration group, Chrysosplenoside A administration group; test drugs were dissolved with 0.5% CMC-NA solution, the dose of 2 g / kg, a single irrigant administration; the mediative control group 0.5% CMC-NA solution, blank control group gave a sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com