Intelligent model for medicine standardization and medicine directory matching
A drug and model technology, applied in the information field, can solve problems such as inability to directly compare specifications and different description forms of specifications, and achieve the effects of wide application range, simple maintenance, and less data storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
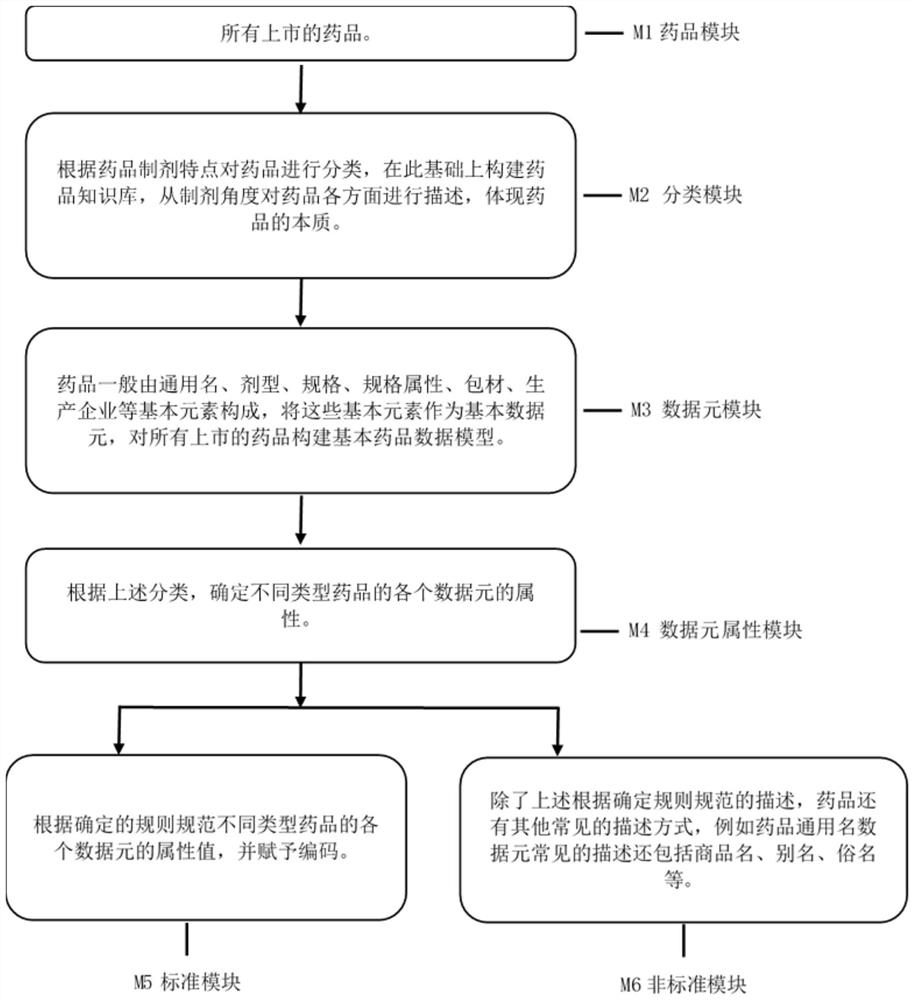
[0046] refer to figure 1 , the drug knowledge base construction related to the intelligent model includes the following modules:
[0047] M1 drug module: all listed drugs;
[0048] From the State Drug Administration, including domestic and imported drugs;
[0049] M2 classification module: Classify drugs according to the characteristics of drug preparations, build a drug knowledge base on this basis, describe all aspects of drugs from the perspective of preparations, and reflect the essence of drugs;
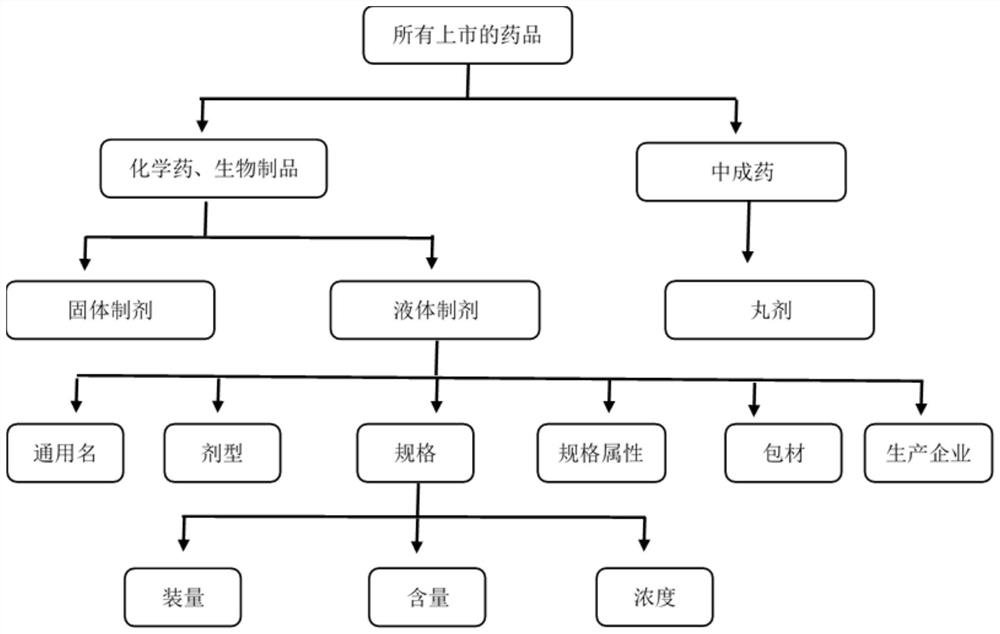
[0050] Firstly, according to the classification of chemical drugs, biological products, and Chinese patent medicines; secondly, according to the classification of preparation forms, drugs are divided into solid preparations, liquid preparations, creams, patches, pills, etc., for example: tablets and capsules are solid preparations, oral liquids , Mixture belongs to liquid preparation, see figure 2 ;
[0051] M3 data element module: Drugs are generally composed of basic elemen...
Embodiment 2
[0066] refer to Figure 4 , the drug standardization intelligent model includes the following modules:
[0067] M1 identifier module: select the generic name, dosage form, specification, specification attribute, packaging material, and the attribute value of the manufacturer data element in the drug knowledge base as the identifier of each element of the drug, including standard description and non-standard description; each data element The attribute values of different data elements are combined into the identification phrase of the drug, the attribute values of the same data element are OR relationship, and the attribute values of different data elements are AND relationship
[0068] Figure 5 The generic name of traditional Chinese medicine 31 is identified by azithromycin, the identified specification is 0.125g or 12.5wiu, the dosage form is hard capsule or capsule, and the manufacturer is identified by Xi’an Daheng Pharmaceutical Co., Ltd. or Xi’an Daheng , then ...
Embodiment 3
[0086] refer to Figure 7 , the drug catalog matching intelligent model includes the following modules:
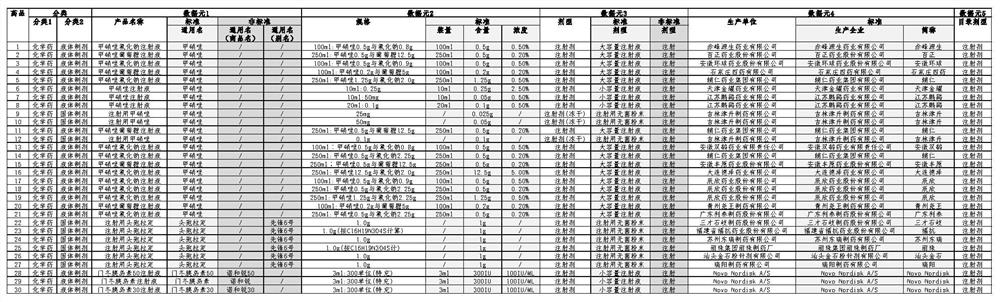
[0087] M1 basic data module: Select the relevant data in the drug knowledge base as the basic data of the model, including classification module, data element module, data element attribute module, etc. The data model of the drug is established on the basis of the classification of drug preparations, from various aspects A standardized description of the preparation characteristics of the drug provides a data basis for the comparison of the essence of the drug, see image 3 ;
[0088] M2 analysis catalog module: Determine the range of analytical drugs included in the comparison according to the analysis catalog, and assign the standard ID of the drug knowledge base to the drugs in the catalog through the drug standardization intelligent model;
[0089] For example, analysis catalog A contains drugs with standard IDs 1-12, see image 3 ;
[0090] M3 reference catalog mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com