New process for preparing 2-acetylfuran from furan
A technology of acetylfuran and acetylfuran crude product, applied in directions such as organic chemistry, can solve problems such as yield and purity decline, multiple side reactions, influence product generation, etc., and achieve the effects of improving utilization rate, improving conversion rate, and improving catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
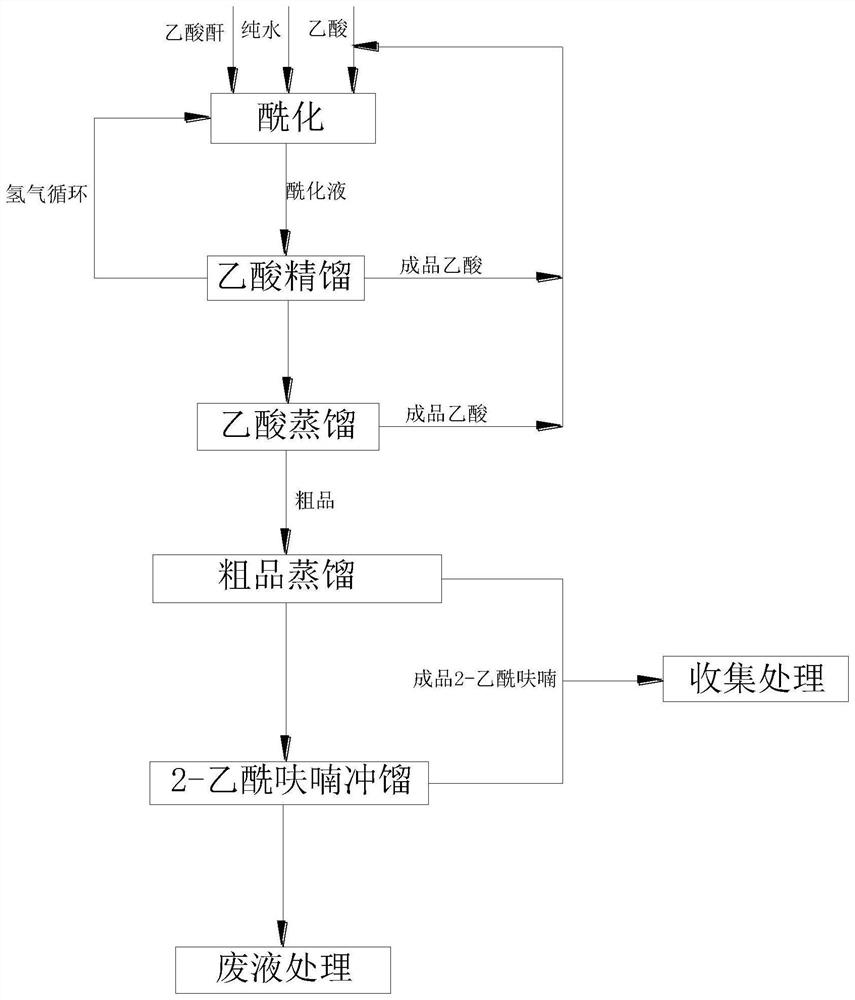
[0032] A new process for preparing 2-acetylfutyofuran using furan, including the following steps:
[0033] Step S1: furanization
[0034] Acetic acid, furan, acetic anhydride and water were added to the esterification reaction kettle, and the solid catalyst was added and uniform, and the esterification reaction was carried out at 30 ° C, pressure + 0.05 MPa, and the esterified reaction liquid, wherein acetic acid, furan is obtained. , The proportion of adding mass of acetic anhydride and solid catalyst is 1: 1: 5: 0.1;
[0035] Step S2: Esterified reaction liquid circulation
[0036] The esterification reaction liquid in which the esterified reaction kettone bottom is packed into the circulating condenser, and then introduced into the esterification reaction kettle after cooling temperature drop to -10 ° C, circulating reaction;
[0037] Step S3: Refining of the esterified reaction liquid
[0038] After the esterification reaction, the esterified reaction liquid was subjected to p...
Embodiment 2
[0043] A new process for preparing 2-acetylfutyofuran using furan, including the following steps:
[0044] Step S1: furanization
[0045] Acetic acid, furan, acetic anhydride and water were added to the esterification reaction kettle, and the solid catalyst was added and uniformly added, and the esterification reaction was carried out at 45 ° C, pressure + 0.05 MPa, and the esterified reaction liquid, wherein the acetic acid, furan is obtained. The ratio of the addition quality ratio of acetic anhydride and solid catalyst is 3: 1.5: 8: 0.5;
[0046] Step S2: Esterified reaction liquid circulation
[0047] The esterification reaction liquid in which the esterified reaction kettone bottom is pumped into the circulating condenser, and then introduced into the esterification reaction kettle, circulating reaction;
[0048] Step S3: Refining of the esterified reaction liquid
[0049] After the esterification reaction, the esterification reaction liquid was subjected to pressure-reduced ...
Embodiment 3
[0054] A new process for preparing 2-acetylfutyofuran using furan, including the following steps:
[0055] Step S1: furanization
[0056]Acetic acid, furan, acetic anhydride and water were added to the esterified reaction kettle, and the solid catalyst was added and uniform, and the esterification reaction was carried out at 60 ° C, pressure + 0.05 MPa, and the esterified reaction liquid was obtained, wherein the acetic acid, furan is obtained. , The proportion of the addition mass of acetic anhydride and solid catalyst is 5: 2: 10: 1;
[0057] Step S2: Esterified reaction liquid circulation
[0058] The esterification reaction liquid in which the esterified reactor bottom is obtained by obtaining step S1, and then introduces the esterification reaction kettle, circulating reaction in the chemical renovation of 30 ° C.
[0059] Step S3: Refining of the esterified reaction liquid
[0060] After the esterification reaction, the esterified reaction solution was subjected to pressure-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com