Anti-PD1 and TGF beta bifunctional antibody and preparation method thereof, and pharmaceutical composition containing same
A bifunctional antibody, PD-1 technology, applied in the direction of drug combinations, chemical instruments and methods, botany equipment and methods, etc., can solve the problems of mediocre experimental results and high clinical toxicity, and achieve good specificity and low in vivo toxicity , the effect of improving the clinical response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1. Expression of fusion protein PD-1 / TGFβRII trap
[0077] Using the extracellular domain of the TGFβRII receptor (SEQ ID NO: 1) as the immunomodulatory molecular part of the fusion protein, the PD-1-op antibody targeting PD1 (secreted anti-PD-1κ light chain polypeptide sequence and heavy chain The polypeptide sequences are shown in SEQ ID NO:2 and SEQ ID NO:3), PD-1-76-C2 antibody, PD-1-97-C2 antibody, PD-1-112-C2 antibody (the constant regions are all Shown in SEQ ID NO:73 and 74) respectively as the targeting part of the fusion protein, with (Gly 4 Ser) 4 Gly is used as a linking sequence to connect the C-terminal of the sequence shown in SEQ ID NO:3 with the N-terminal of the sequence shown in SEQ ID NO:1 to form four fusion proteins. These four proteins have basically the same experimental performance. The following is the same as PD-1 -op antibody fusion formation is represented by Anti-PD1-TGFβRII extracellular region fusion protein, indicating its expe...
Embodiment 2
[0080] Example 2. Expression of fusion protein PD-1 / TGFβRII trap
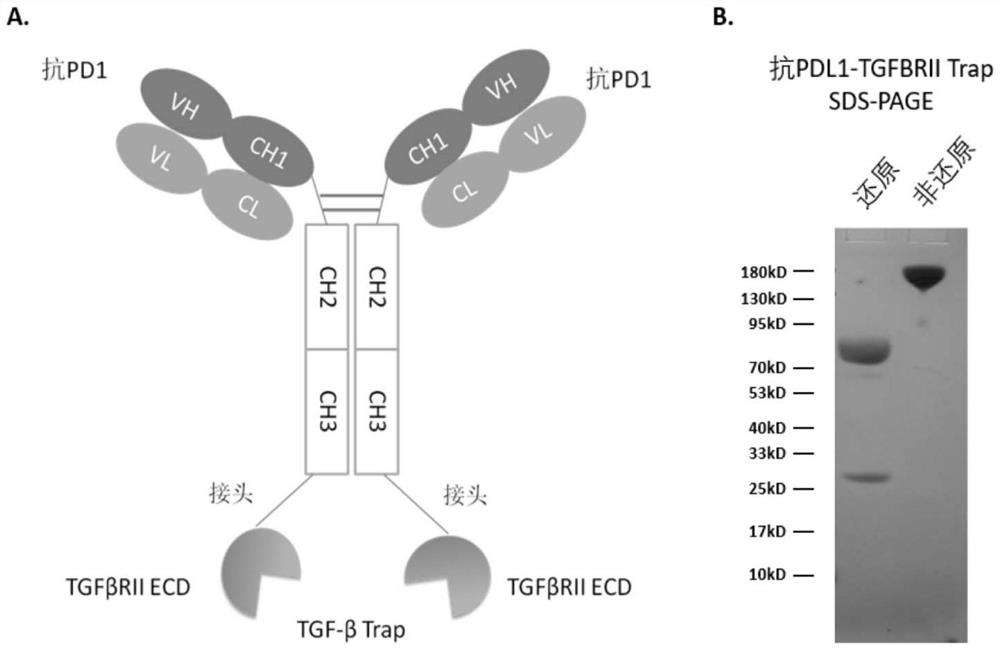
[0081] The expression plasmid was constructed to transiently transfect human embryonic kidney HEK 293 cells, and the anti-PD1-TGFβRIITrap produced by the isolated and purified cells had a band molecular weight of about 170kD on SDS-PAGE under non-reducing conditions ( figure 1 In the B panel), the molecular weight of the band on SDS-PAGE under reducing conditions is about 80kD. There was a small peak after the 190kD peak on size exclusion chromatography, which was identified by mass spectrometry as the anti-PD1-TGFBR2 trap antibody moiety cleaved at a site in the N-terminal portion of TGFβRII.
Embodiment 3
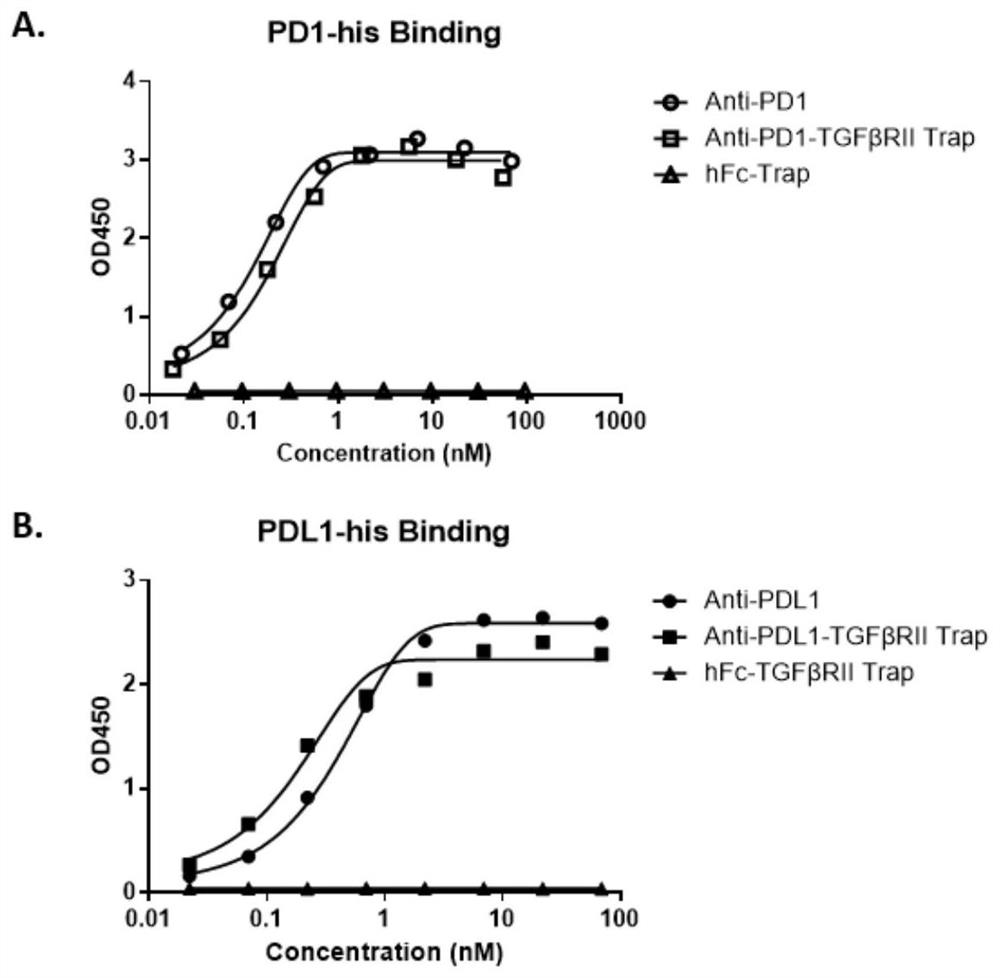
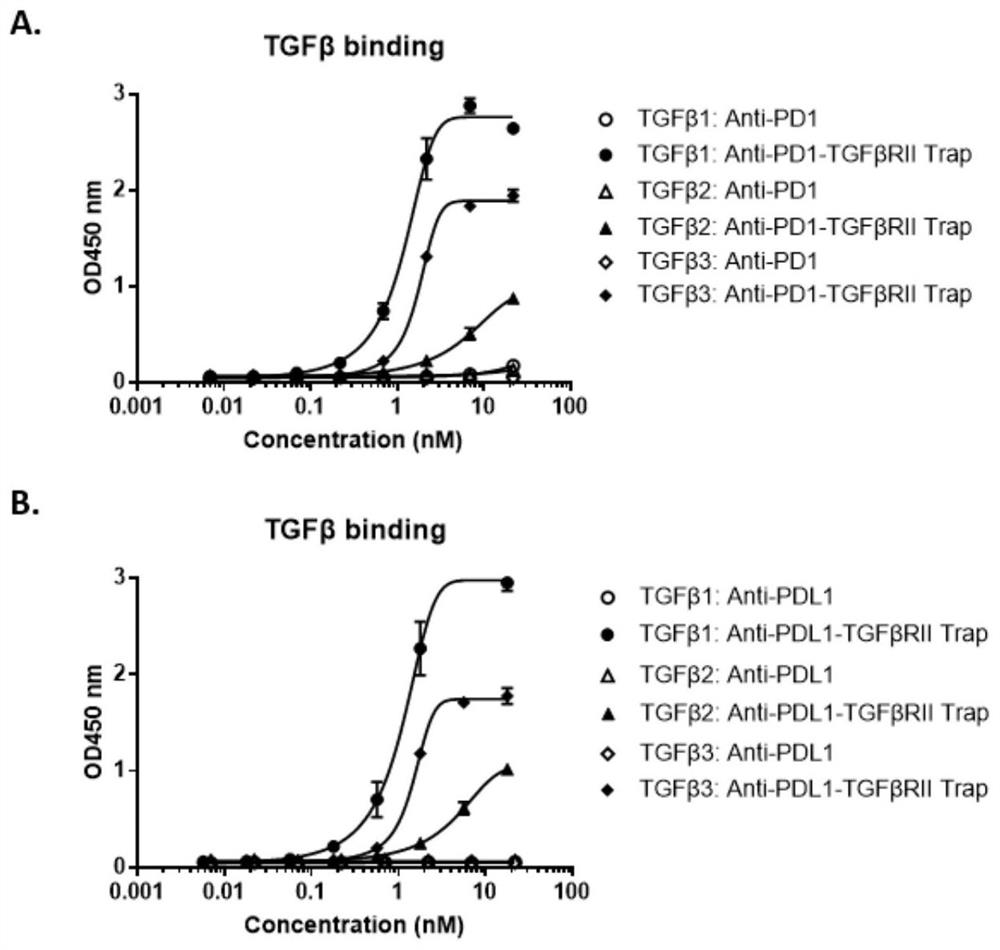
[0082] Example 3. ELISA detection of binding activity of PD1-TGFβRII trap antibody end and trap end in vitro
[0083] The antigens used for the binding detection of antibody end and trap end are self-produced PD1-His (SEQ ID NO: 12), PDL1-His (SEQ ID NO: 13), human TGFβ1 (CA59), human TGFβ2 (CJ79), human TGFβ3 (CJ44) (TGFβ protein was purchased from Novoprotein), and the detection process was as follows:
[0084] Dilute PD1-His (SEQ ID NO:12), TGFβ1, TGFβ2, TGFβ3 to 0.5 μg / ml with 1× phosphate buffered saline (PBS), coat 96-well microtiter plate with 100 μl / well, overnight at 4°C;
[0085] Wash 3 times with 250 μl 1×PBST (PBS+0.5% Tween20), add 200 μl PBS containing 2% bovine serum albumin (BSA) to block for 1 hour at room temperature;
[0086] Wash 3 times with 250 μl 1×PBST, add serially diluted PD1-TGFβRII trap, PD1 antibody as positive control, incubate at room temperature for 2 hours;
[0087] Wash 3 times with 250 μl 1×PBST, add 100 μl diluted Goat-anti-human Fc-HRP co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com