Polymer composition for non-aqueous secondary batteries, and non-aqueous secondary battery
A secondary battery, non-aqueous technology, applied in non-aqueous electrolyte battery electrodes, secondary batteries, secondary battery repair/maintenance, etc., can solve the problem of insufficient adhesion between copolymer latex and current collectors or separators, Insufficient adhesion, damage to the charge-discharge cycle characteristics of secondary batteries, etc., to achieve good cycle characteristics and good initial capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
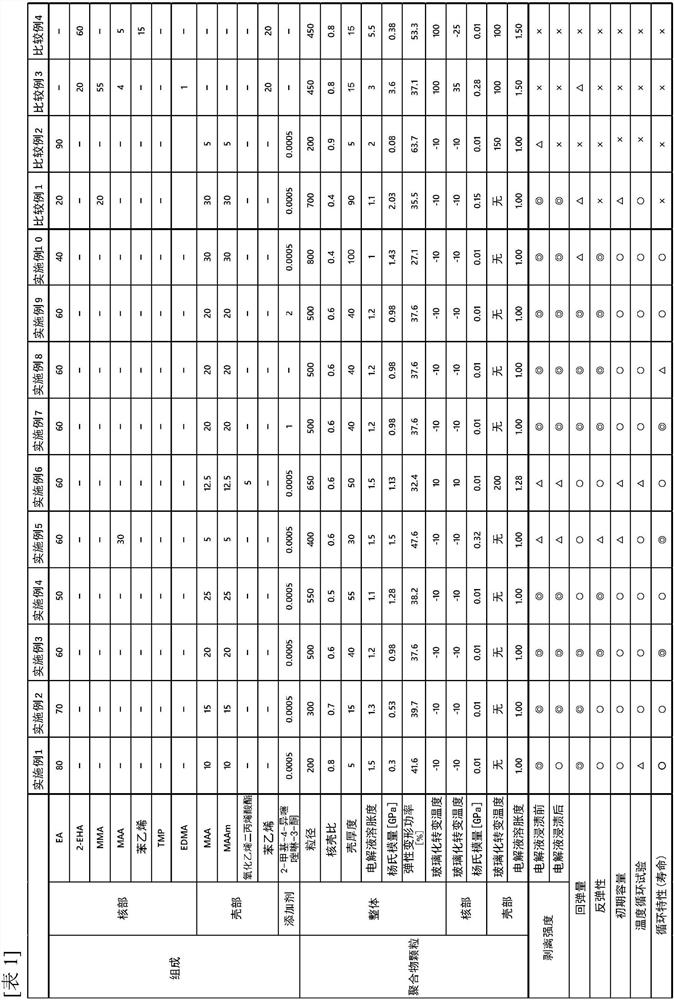
[0126] Ion-exchanged water was added to the reactor, and the temperature was raised and kept at 65° C. while stirring. After adding sodium persulfate (hereinafter also referred to as "NPS") as a polymerization initiator thereto, methacrylic acid (hereinafter also referred to as "monomer") as a monomer (hereinafter also referred to as "monomer") component was added dropwise. "MAA") and methacrylamide (hereinafter also referred to as "MAAm") dissolved in ion-exchanged water, and a 10% sodium hydroxide aqueous solution were used for 2 hours while maintaining the temperature at 65°C. After adding dropwise, polymerization was continued for 1 hour. Next, ethyl acrylate (hereinafter also referred to as "EA") was added dropwise, and polymerization was continued for 1 hour. As the mixing amount of each component at this time, when the total of the monomer components (units derived from all ethylenically unsaturated monomers: EA, MAA, MAAm) is 100 parts by mass, EA is 80 parts by mass,...
Embodiment 2~10 and comparative example 1~2
[0136] In each example, the compounding quantity of a monomer and 2-methyl-4-isothiazolin-3-one was changed as shown in Table 1.
Embodiment 5
[0137] In Example 5, MAA was also added in the compounding amount shown in Table 1 at the time of adding EA as a monomer constituting the core.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com