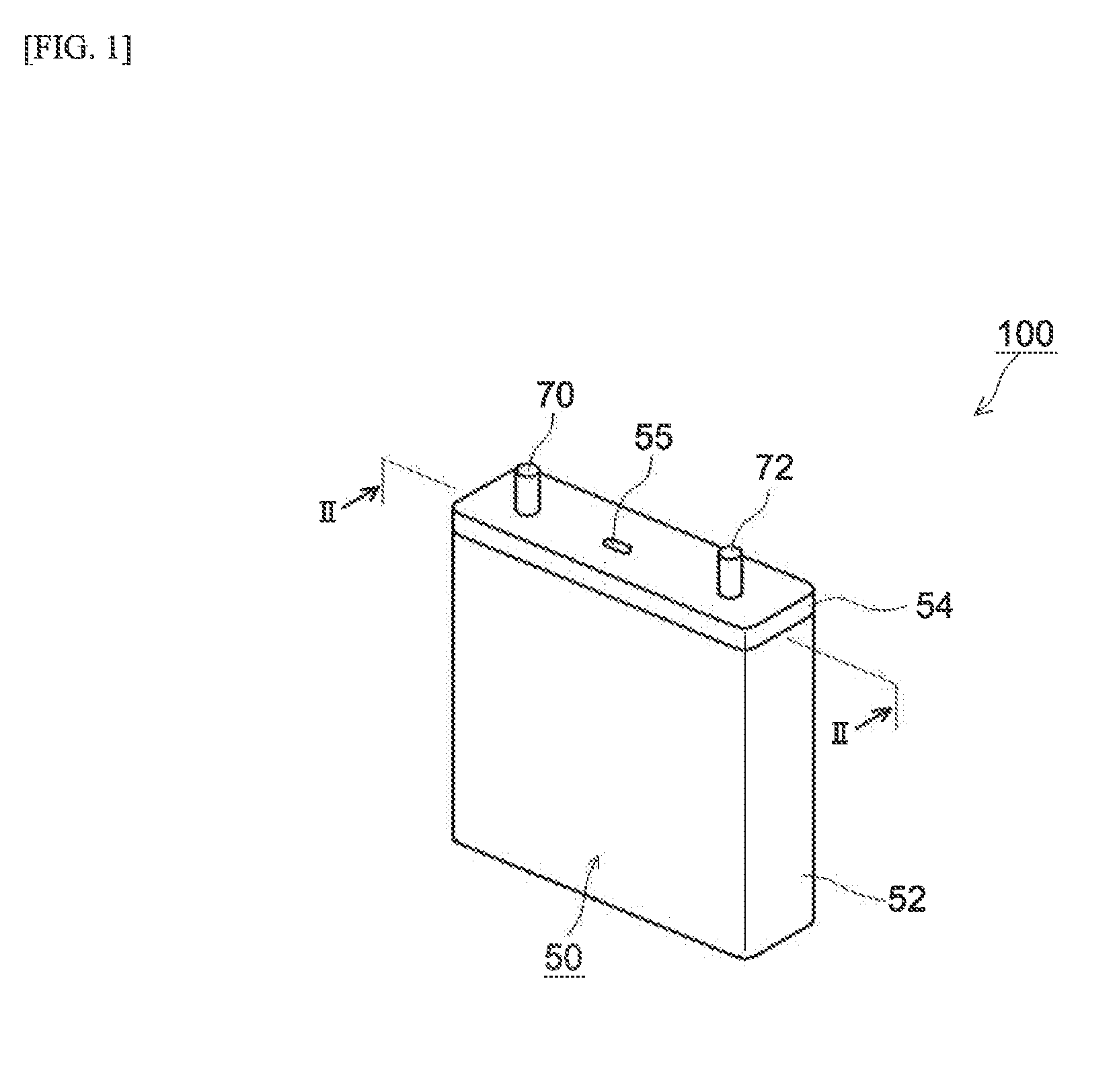
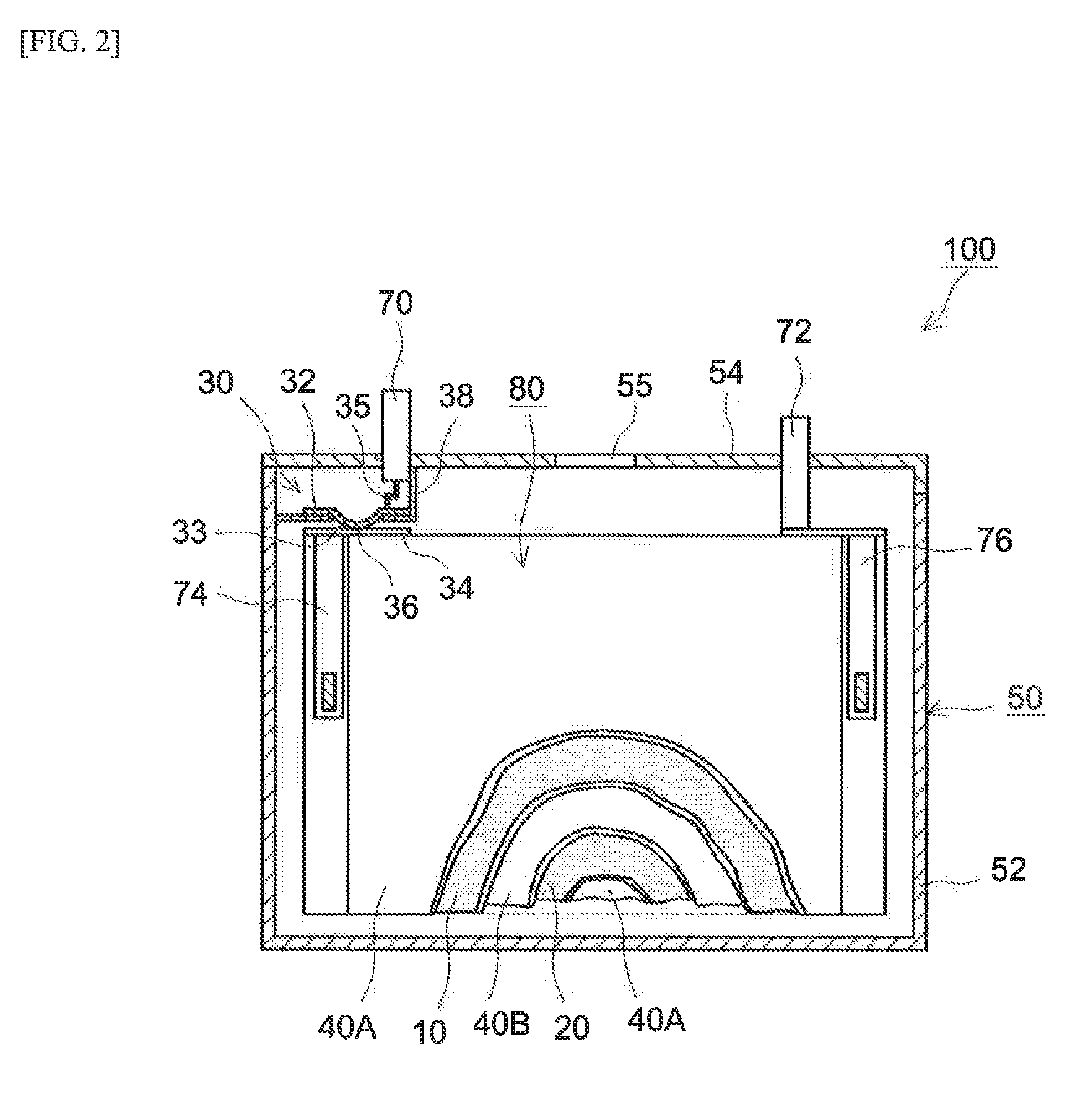
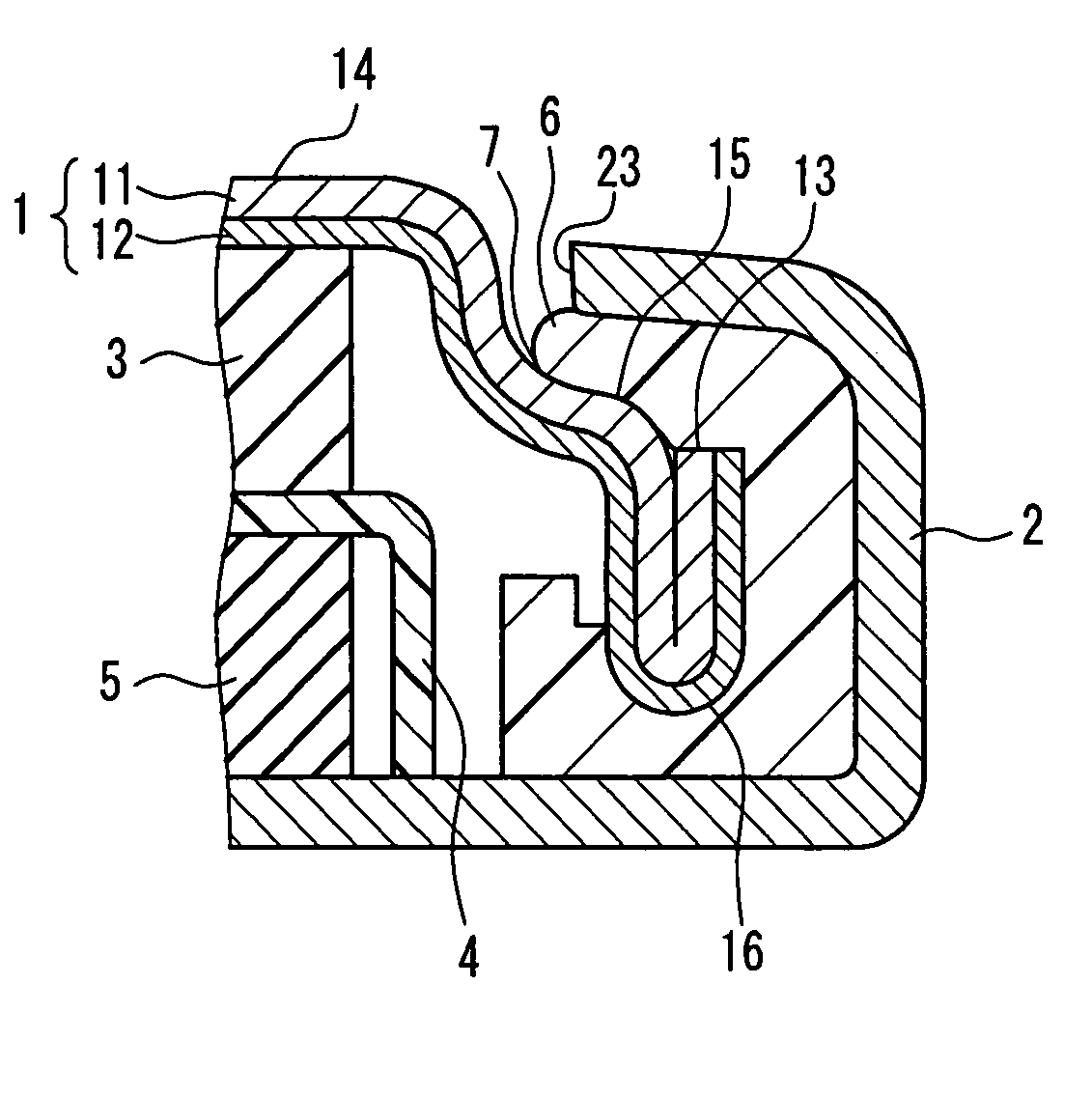
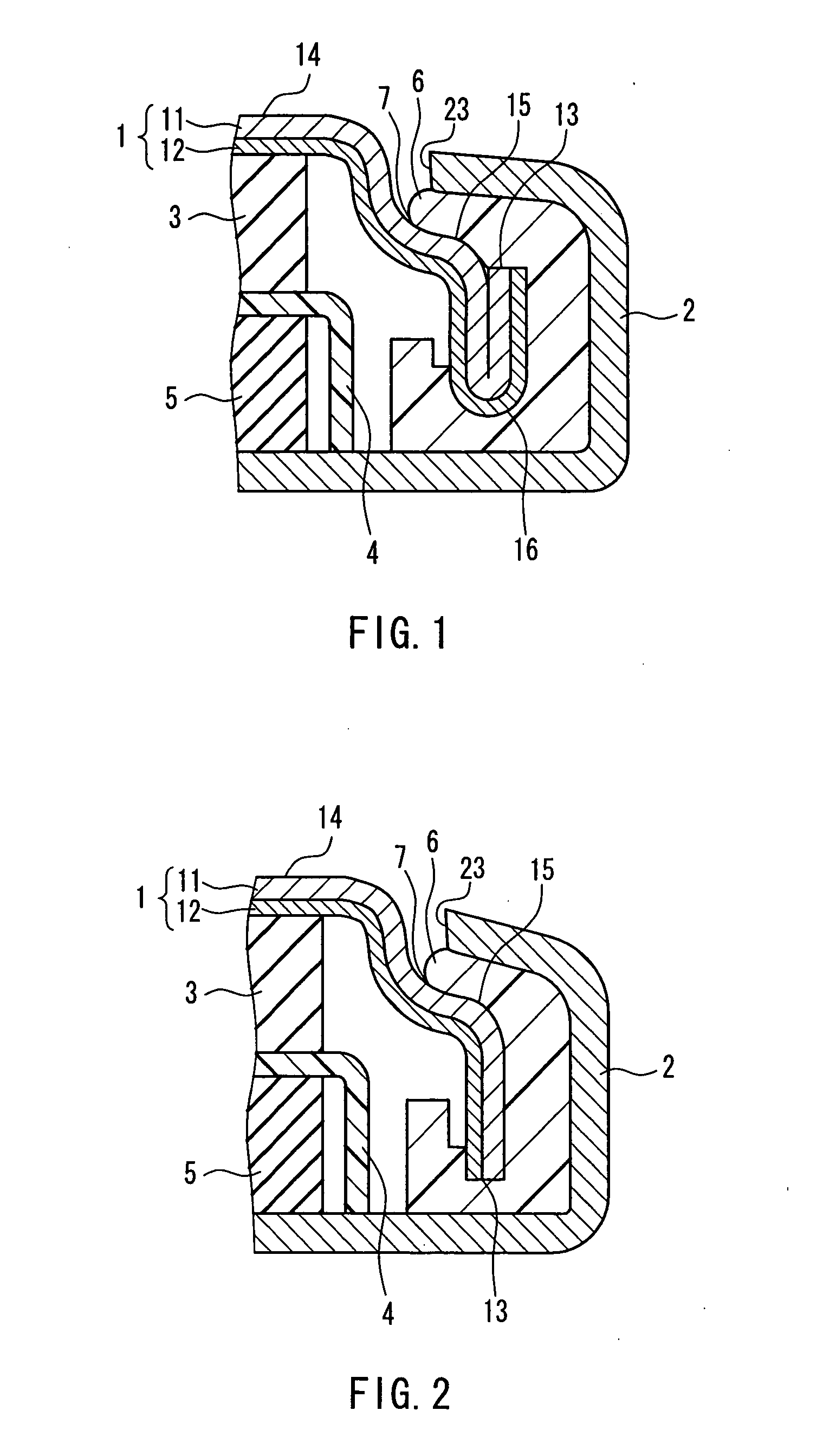
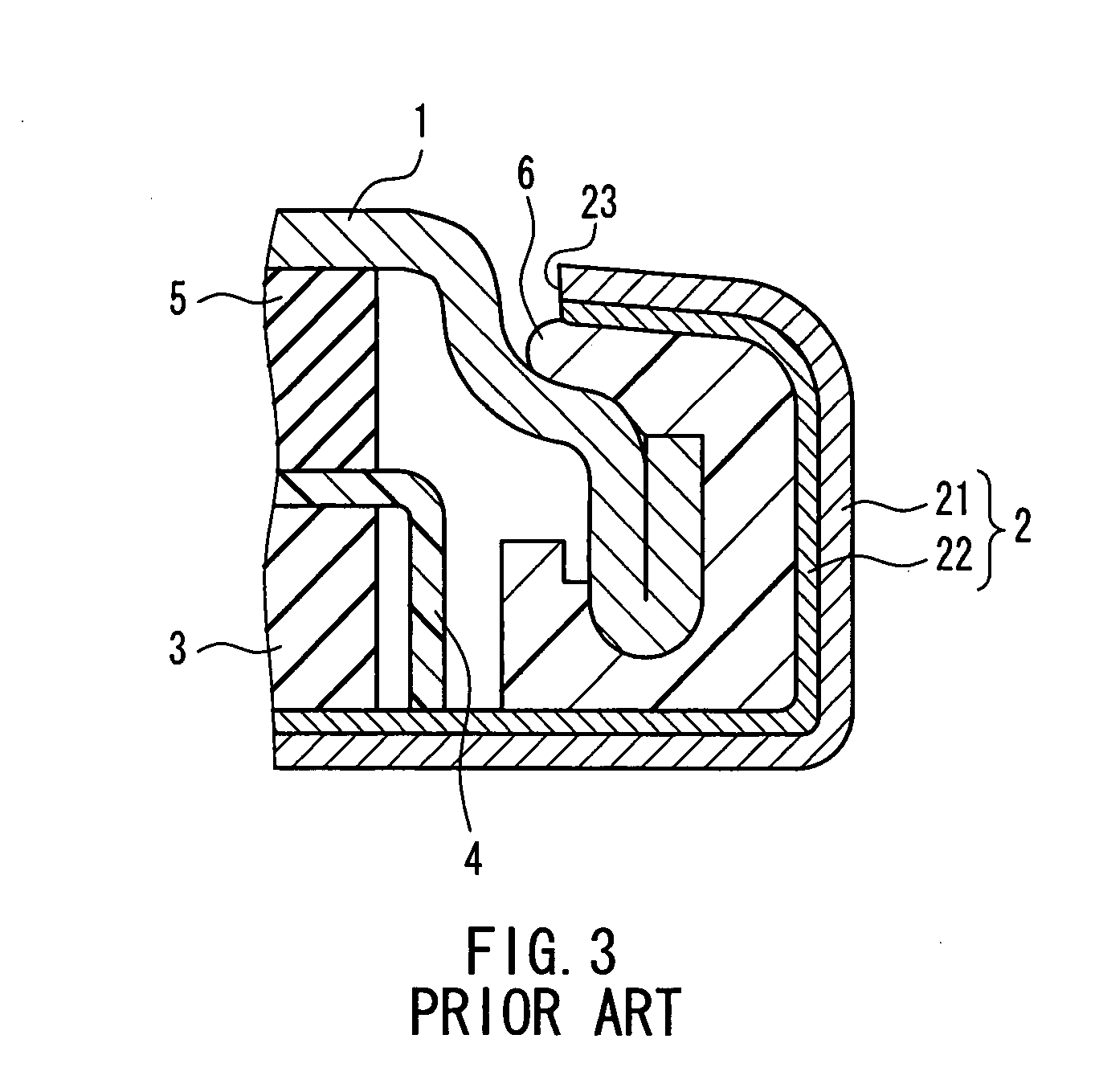
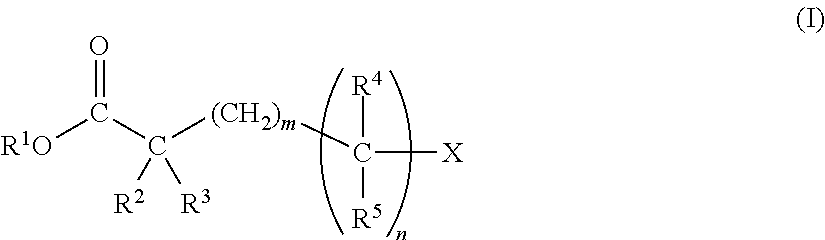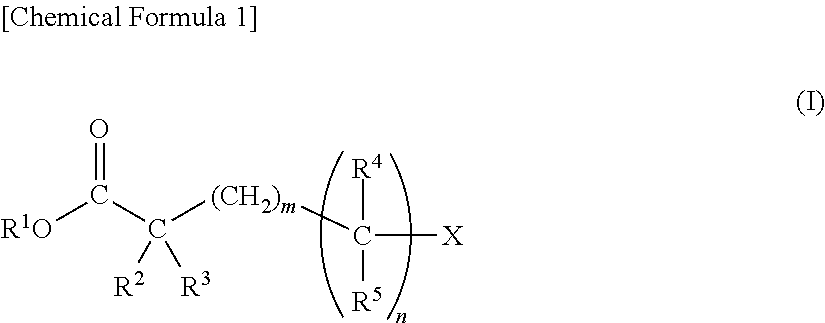Patents
Literature
258results about "Non-aqueous electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stable electrolyte counteranions for electrochemical devices
InactiveUS20070048605A1Desirable chemical stabilityDesirable thermal stabilityConductive materialOrganic electrolyte cellsArylHydrogen
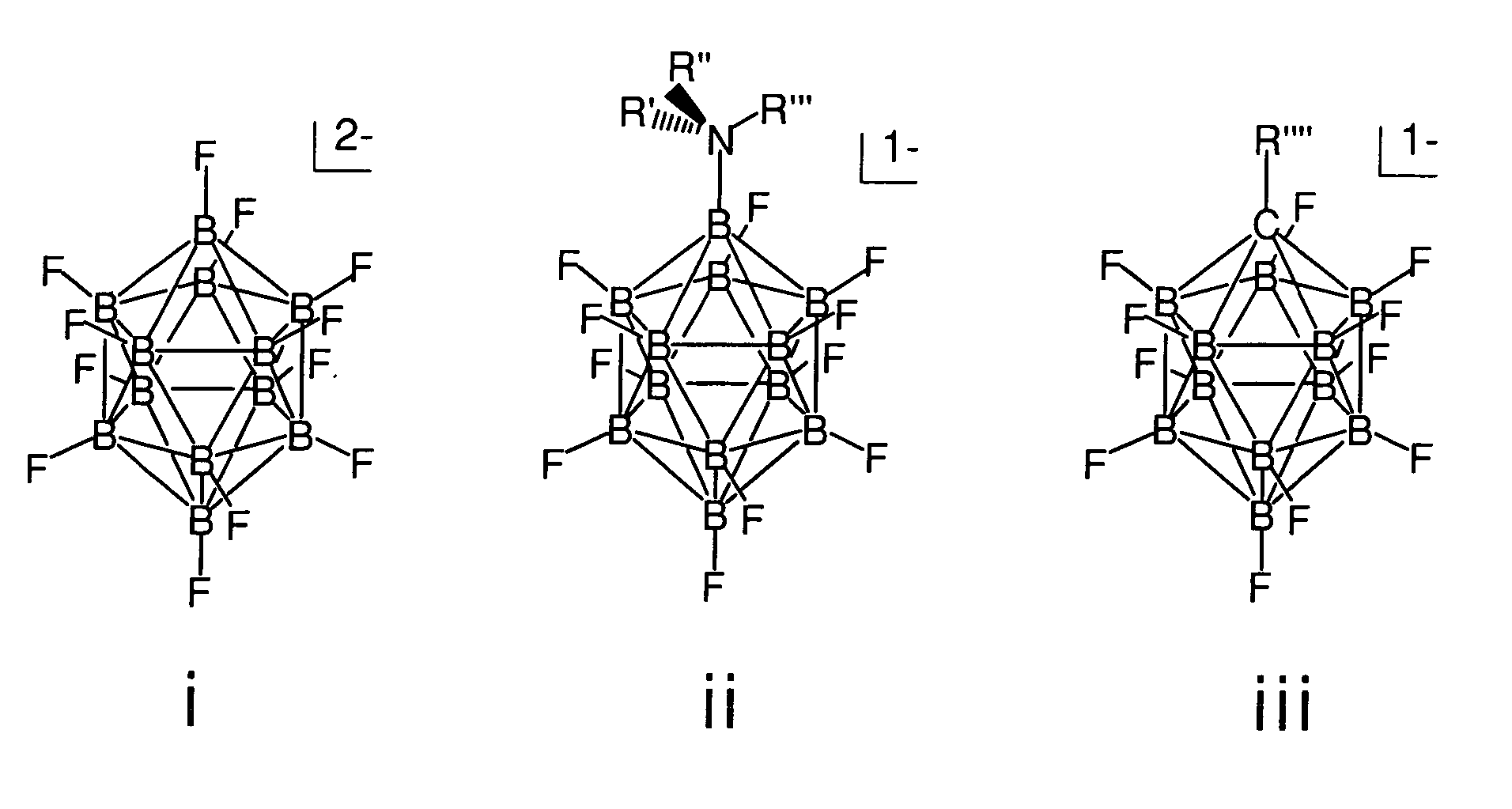
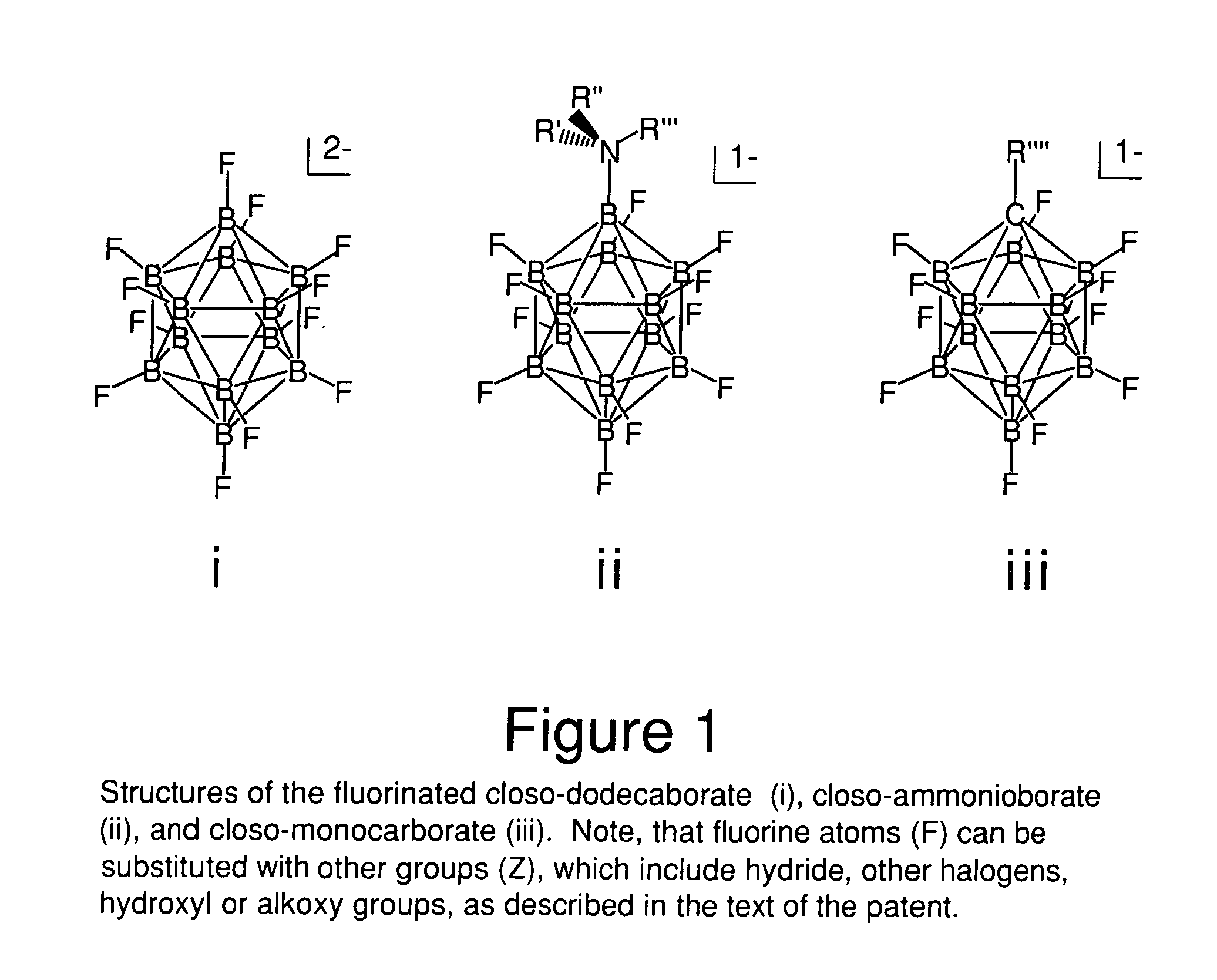
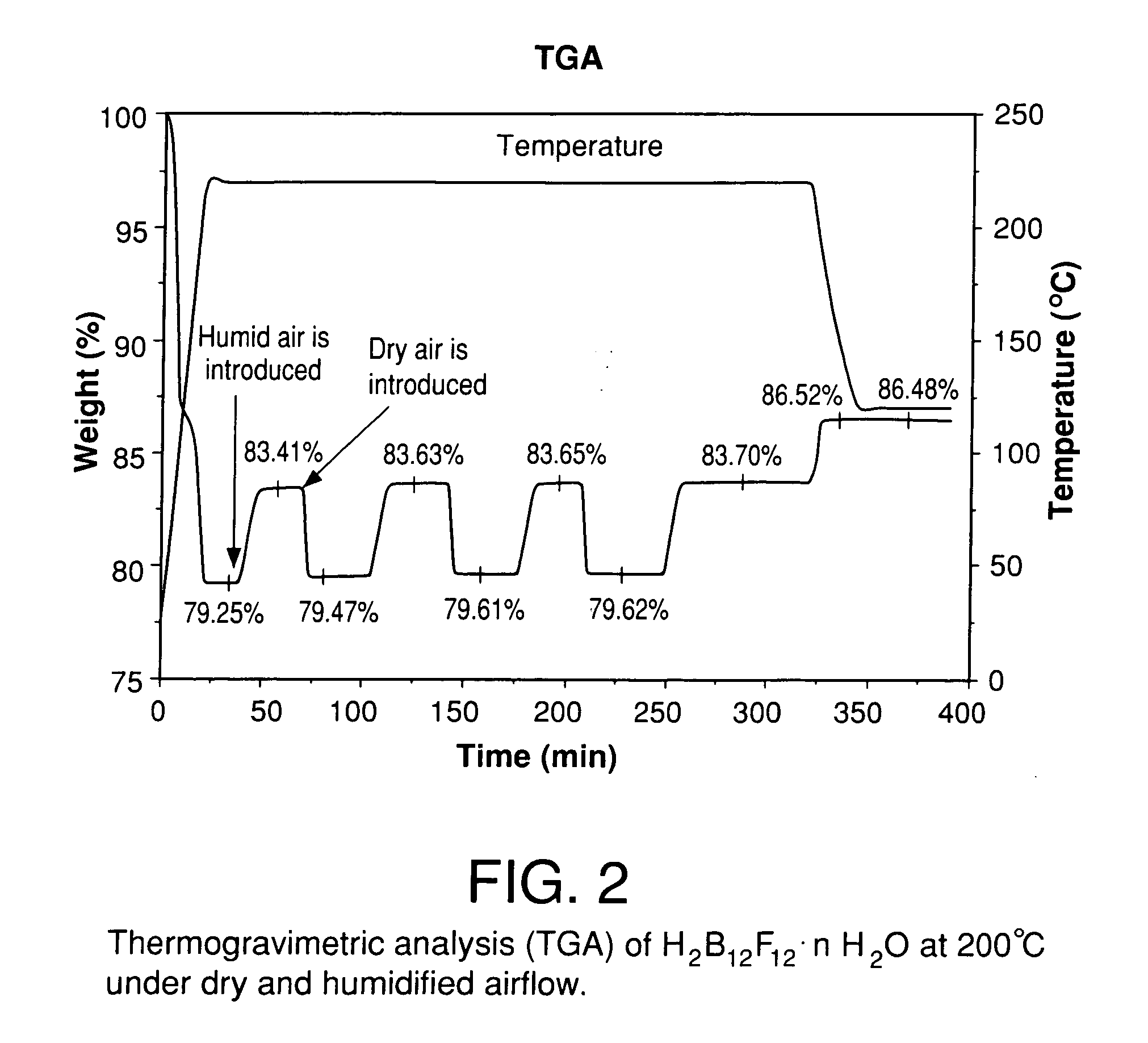
The invention relates to electrolyte salts for electrochemical devices of improved physical, chemical and electrochemical stability. The improvement resides in the use of anions of salts of the formula comprising: i) (B12FxZ12-x)2− wherein Z comprises at least one of H, Cl, Br or OR; R comprises at least one of H, alkyl or fluoroalkyl, or at least one polymer and x is at least 3 on an average basis but not more than 12; ii) ((R′R″R′″)NB12FxZ(11-x))−, wherein N is bonded to B and each of R′, R″, R′″ comprise a member independently selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl and a polymer; Z comprises H, Cl, Br, or OR, where R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11; or iii) (R″″CB11FxZ(11-x))−, wherein R″″ is bonded to C and comprises a member selected from the group consisting of hydrogen, alkyl, cycloalkyl, aryl, and a polymer, Z comprises H, Cl, Br, or OR, wherein R comprises H, alkyl or perfluoroalkyl or a polymer, and x is an integer from 0 to 11.
Owner:AIR PROD & CHEM INC
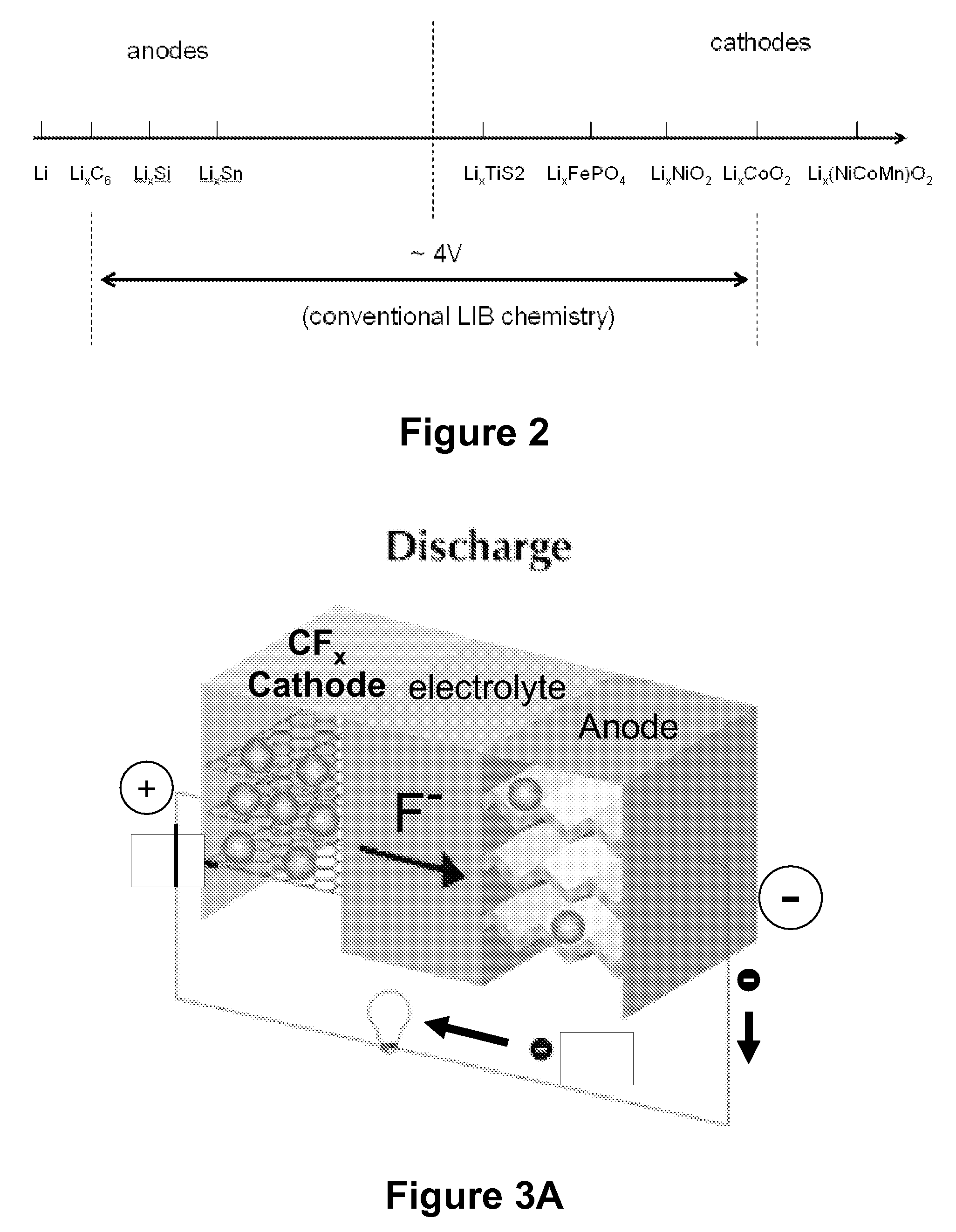
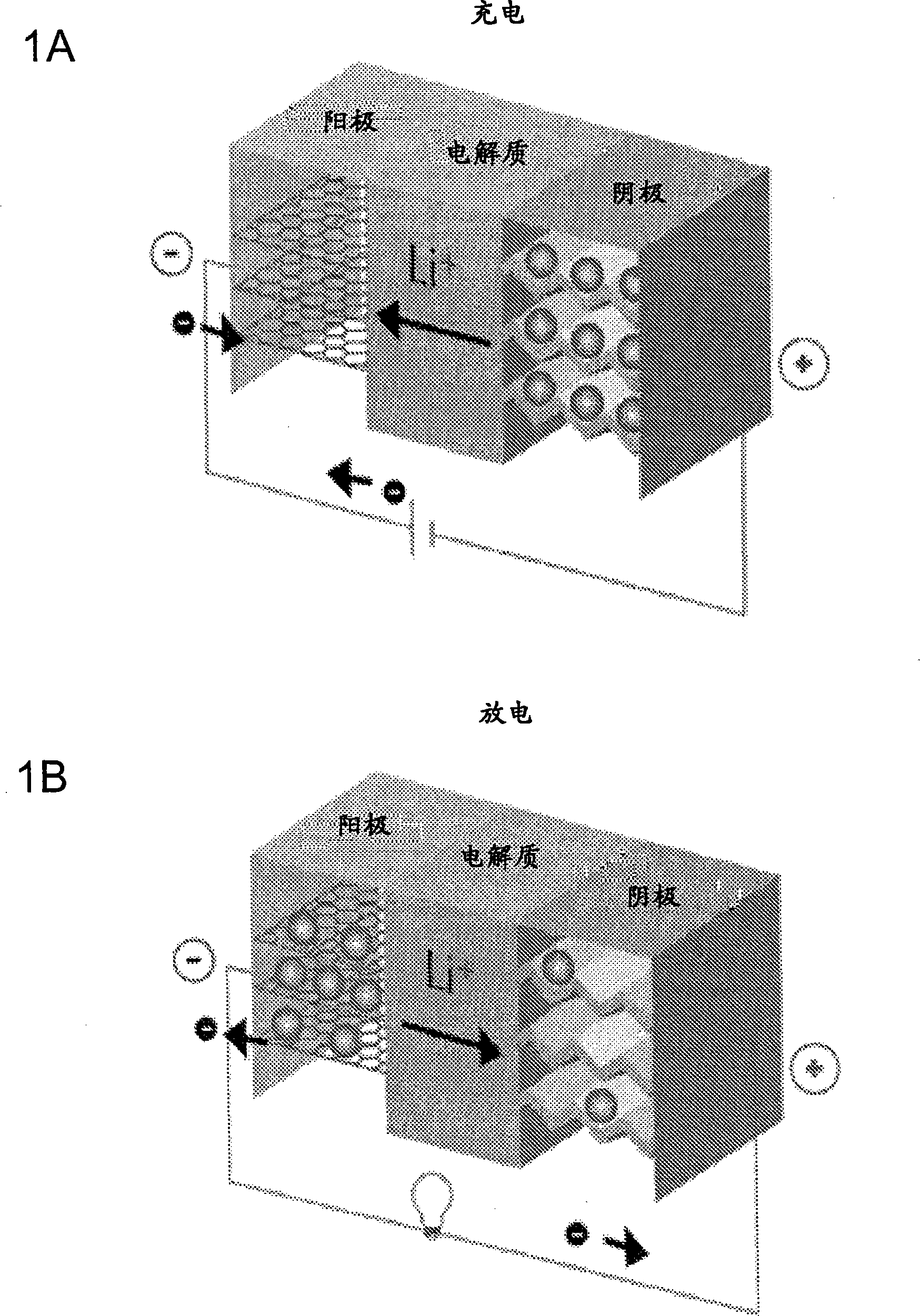
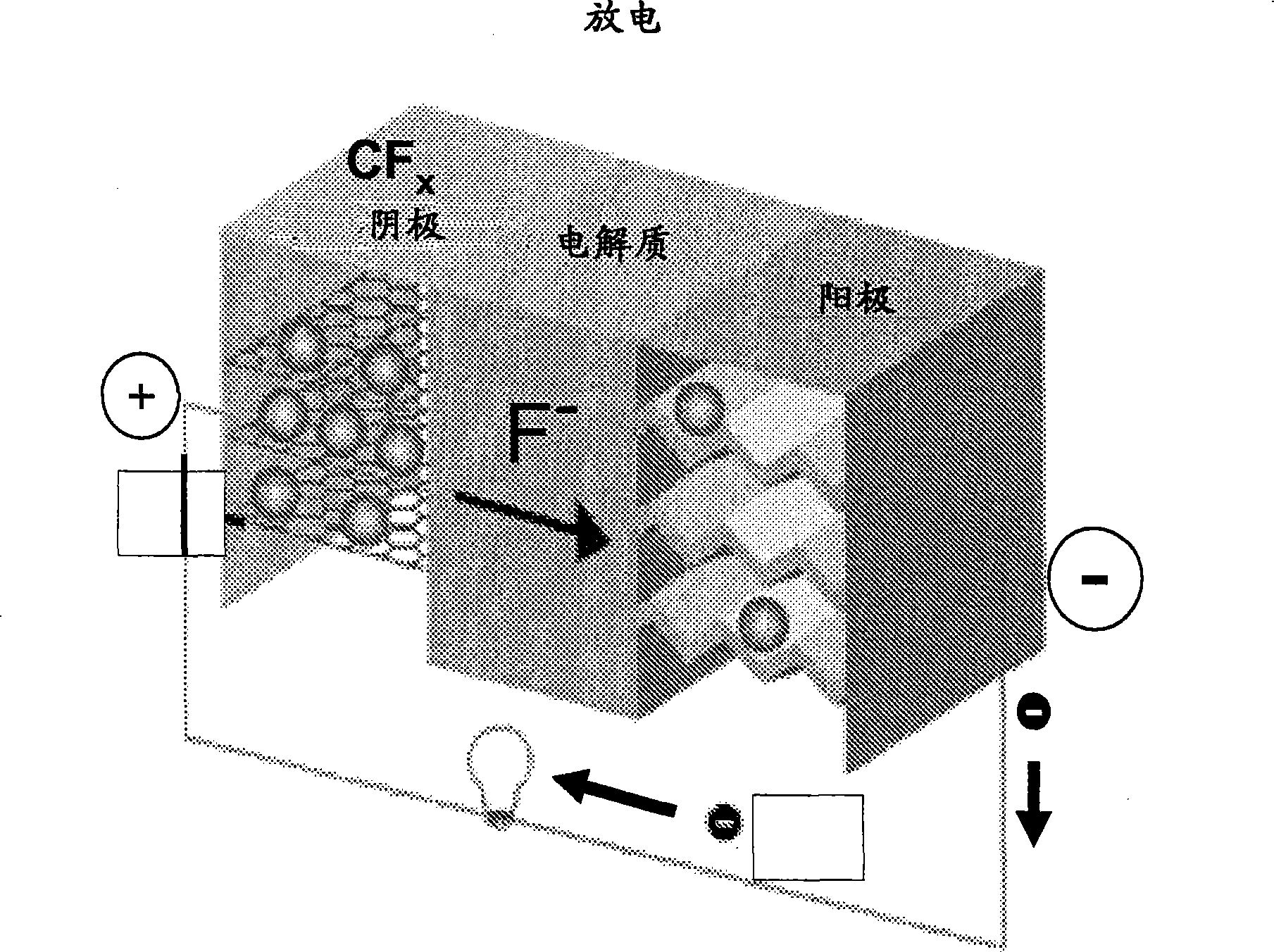
Fluoride ion electrochemical cell
ActiveUS20090029237A1Improve performanceHigh energyAlkaline accumulatorsLead-acid accumulatorsMetallic lithiumState of art
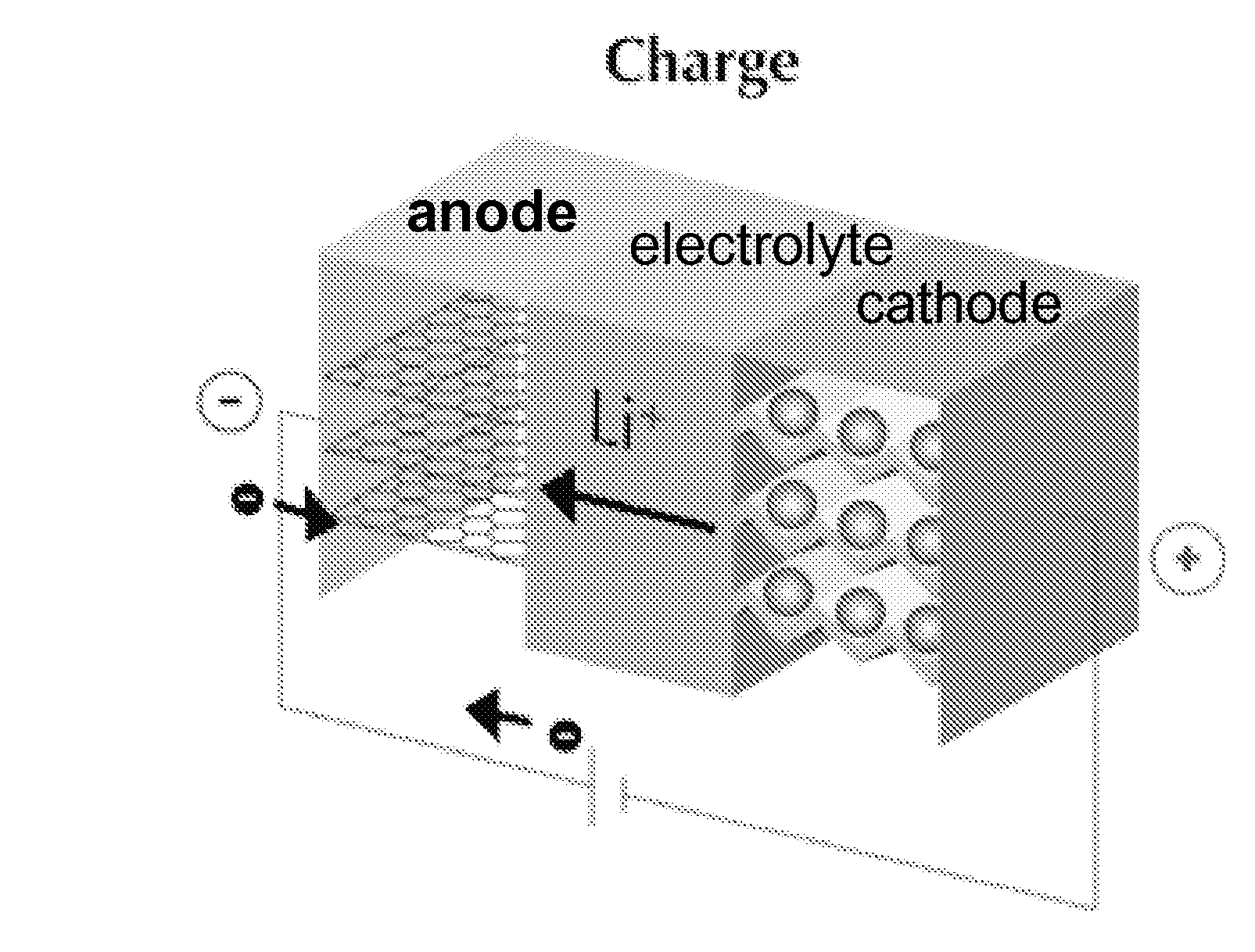
The present invention provides electrochemical cells capable of good electronic performance, particularly high specific energies, useful discharge rate capabilities and good cycle life. Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using anion charge carriers capable of accommodation by positive and negative electrodes comprising anion host materials, which entirely eliminate the need for metallic lithium or dissolved lithium ion in these systems.
Owner:CALIFORNIA INST OF TECH +1
Negative electrode material for nonaqueous electrolyte secondary battery, making method and lithium ion secondary battery
InactiveUS20100243951A1High charge-discharge efficiencyImprove cycle performanceMaterial nanotechnologyDecorative surface effectsDischarge efficiencyLithium
A negative electrode material comprising composite particles having silicon nano-particles dispersed in silicon oxide is suited for use in nonaqueous electrolyte secondary batteries. The silicon nano-particles have a size of 1-100 nm. The composite particles contain oxygen and silicon in a molar ratio: 0<O / Si<1.0. Using the negative electrode material, a lithium ion secondary battery can be fabricated which features high 1st cycle charge / discharge efficiency, capacity, and cycle performance.
Owner:SHIN ETSU CHEM IND CO LTD
Battery separator and nonaqueous electrolyte battery
ActiveUS20110052987A1Heat suppressionImprove securityFinal product manufactureNon-aqueous electrolyte accumulator electrodesLithiumEngineering
A nonaqueous electrolyte battery of the present invention includes a positive electrode having a positive active material capable of intercalating and deintercalating a lithium ion, a negative electrode having a negative active material capable of intercalating and deintercalating a lithium ion, a separator interposed between the positive electrode and the negative electrode, and a nonaqueous electrolyte. The heat generation starting temperature of the positive electrode is 180° C. or higher. The separator includes heat-resistant fine particles and a thermoplastic resin. The proportion of particles with a particle size of 0.2 μm or less in the heat-resistant fine particles is 10 vol % or less and the proportion of particles with a particle size of 2 μm or more in the heat-resistant fine particles is 10 vol % or less. The separator effects a shutdown in the range of 100° C. to 150° C.
Owner:MAXELL HLDG LTD
Nonaqueous Electrolyte Solution and Lithium Secondary Battery Using Same
ActiveUS20090226808A1Large capacityIncreased operating lifeHybrid capacitor electrolytesElectrolytic capacitorsHydrogenHydrogen atom
Disclosed is a nonaqueous electrolyte solution containing a sultone compound represented by the Formula 1 below (wherein R1 to R4 respectively represent a hydrogen, a fluorine, a hydrocarbon group with 1 to 12 carbon atoms that may contain fluorine atom(s), n represents an integer of 0 to 3, and when n is 2 or 3, the two or three R3 groups are independent from each other and the two or three R4 groups are independent from each other), and an ethylene carbonate having a hydrogen atom substituted by a fluorine atom. Also disclosed is a lithium secondary battery employing the nonaqueous electrolyte solution. This nonaqueous electrolyte solution does not cause an increase in the internal resistance of a nonaqueous electrochemical device and improves the lifespan characteristics of the device. The lithium secondary battery containing the nonaqueous electrolyte solution exhibits greatly improved cycle charge / discharge characteristics at high temperature, and has excellent charge / discharge load characteristics.
Owner:MITSUI CHEM INC
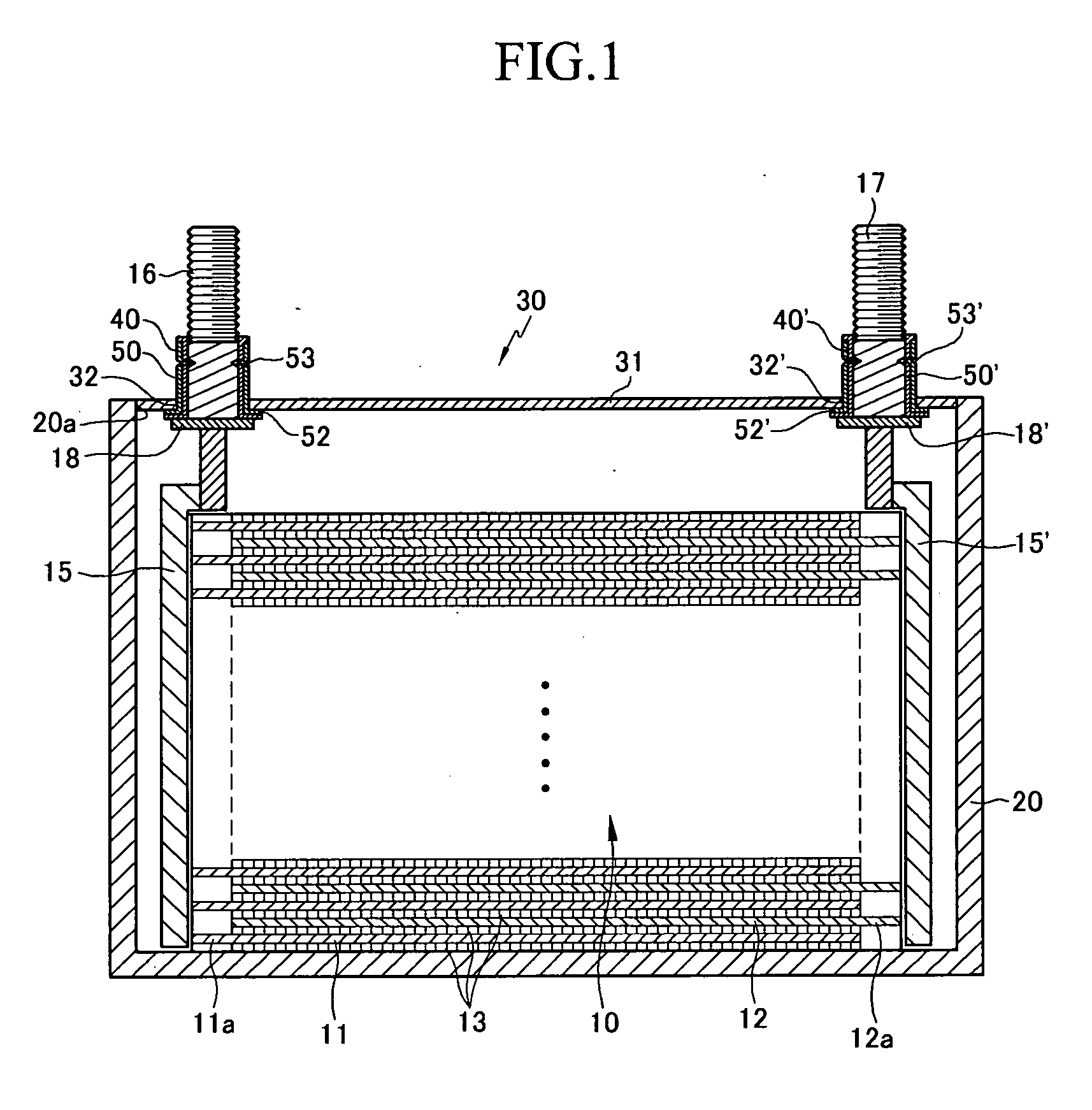
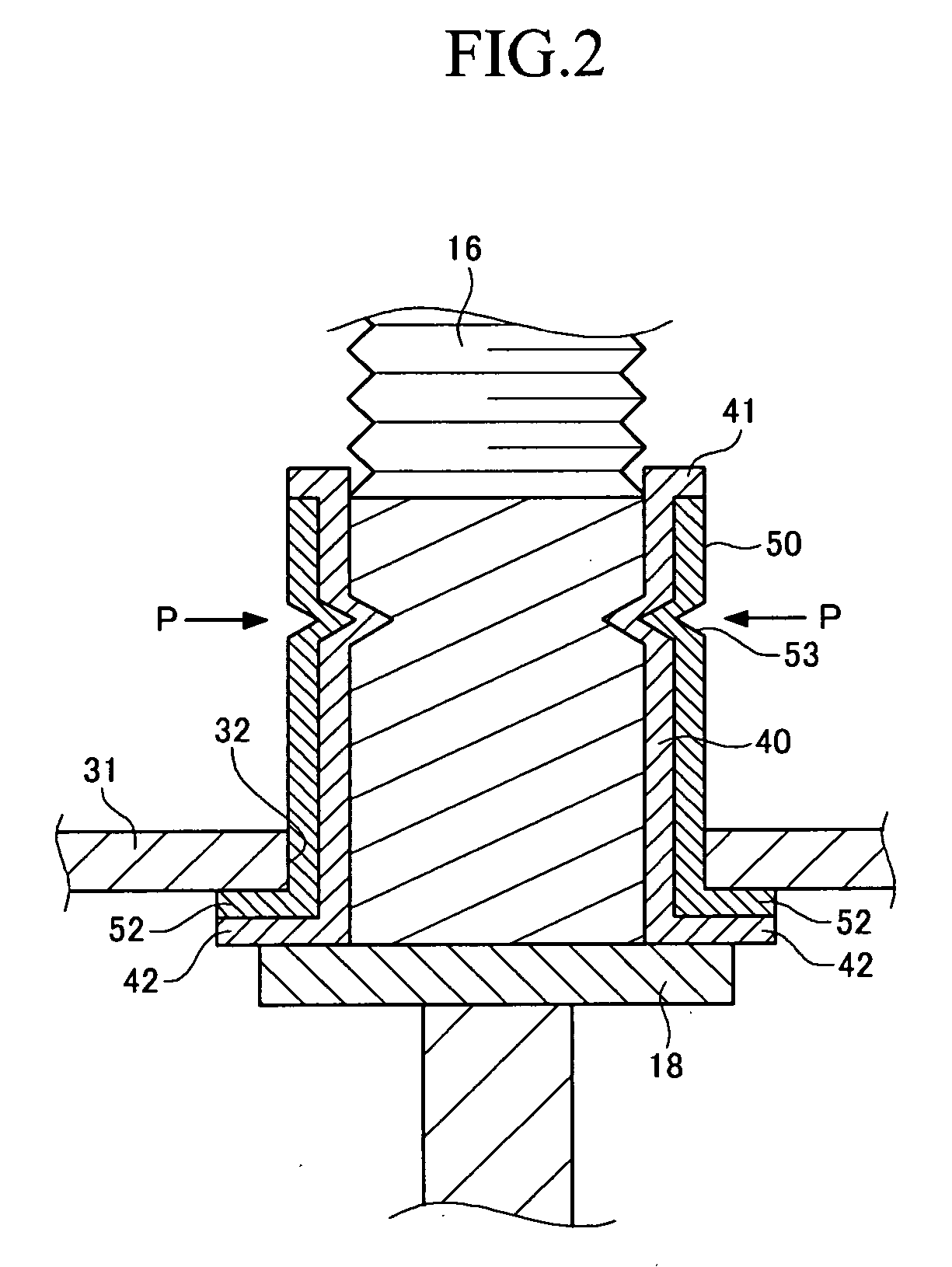
Rechargeable battery
ActiveUS20060115727A1Simplify the assembly processShorten assembly timeLarge-sized flat cells/batteriesFinal product manufactureRechargeable cellElectrical and Electronics engineering
A rechargeable battery includes an electrode assembly including a positive electrode, a negative electrode, and a separator interposed between the two electrodes, a container for receiving the electrode assembly inside, and a cap assembly fixed to the container. The cap assembly includes a cap plate fixed to the container, an external terminal disposed in the cap plate to electrically be coupled with the electrode assembly, and a tubular body that surrounds the external terminal to fix the external terminal to the cap plate. The external terminal includes at least one groove formed toward inside of the external terminal for fastening the external terminal to the tubular body.
Owner:SAMSUNG SDI CO LTD
Lithium Ion Secondary Battery
InactiveUS20080038631A1Improve reliabilityImprove securityFinal product manufactureNegative electrodesLithium oxidePhysical chemistry
A lithium ion secondary battery includes a positive electrode comprising a composite lithium oxide; a negative electrode capable of charge and discharge; a separator; and a non-aqueous electrolyte including a non-aqueous solvent and a solute dissolved therein. The separator includes at least one heat-resistant porous film and at least one shut-down layer. A porous membrane is bonded to a surface of at least one selected from the positive electrode and the negative electrode, and the porous membrane comprises an inorganic oxide filler and a binder.
Owner:PANASONIC CORP
Non-aqueous electrolyte and non-aqueous electrolyte secondary cell
InactiveUS20040091786A1Reduce gas volumeExcellent characteristicsNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsPhysical chemistryDiphenyl disulfide
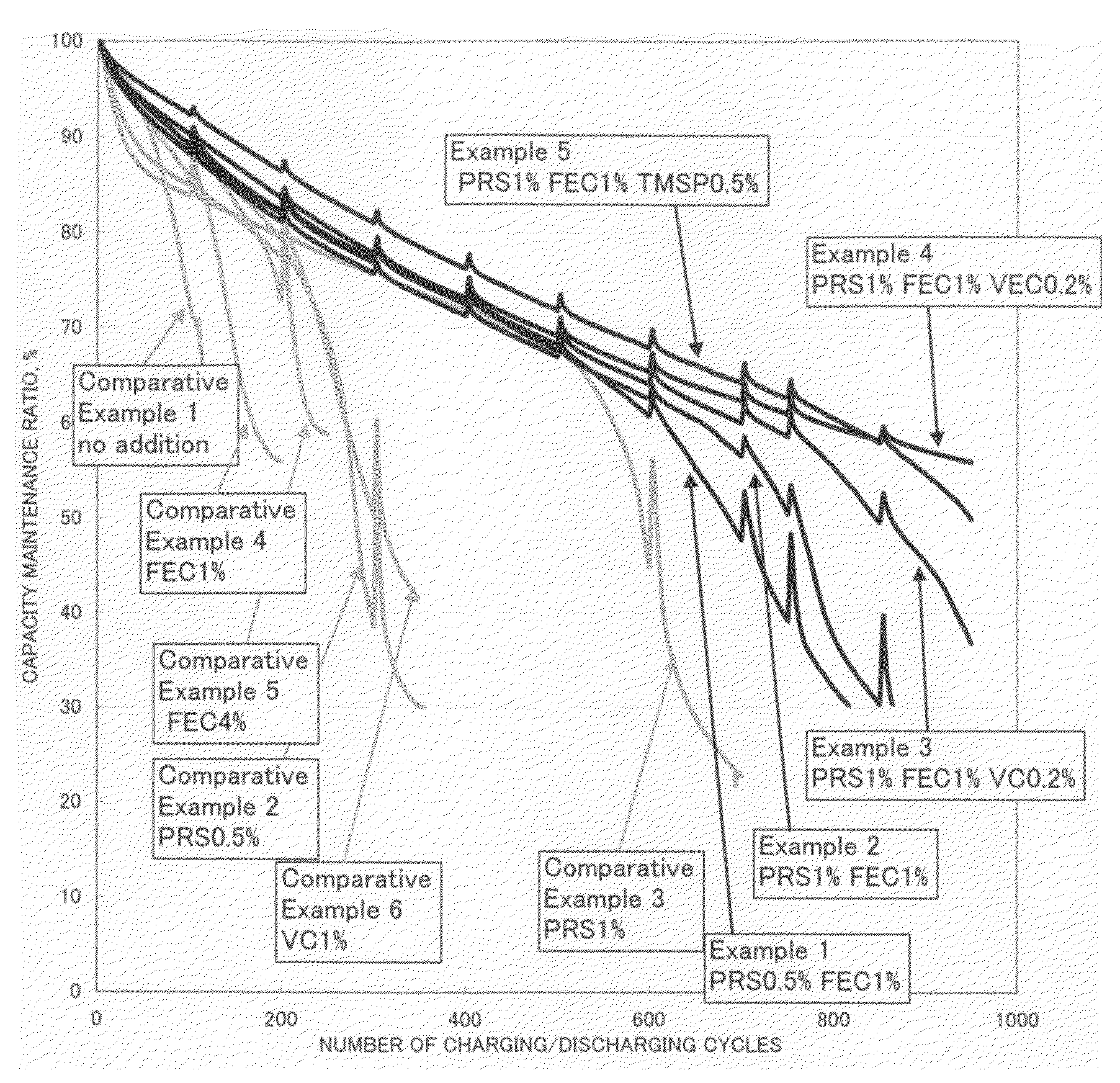
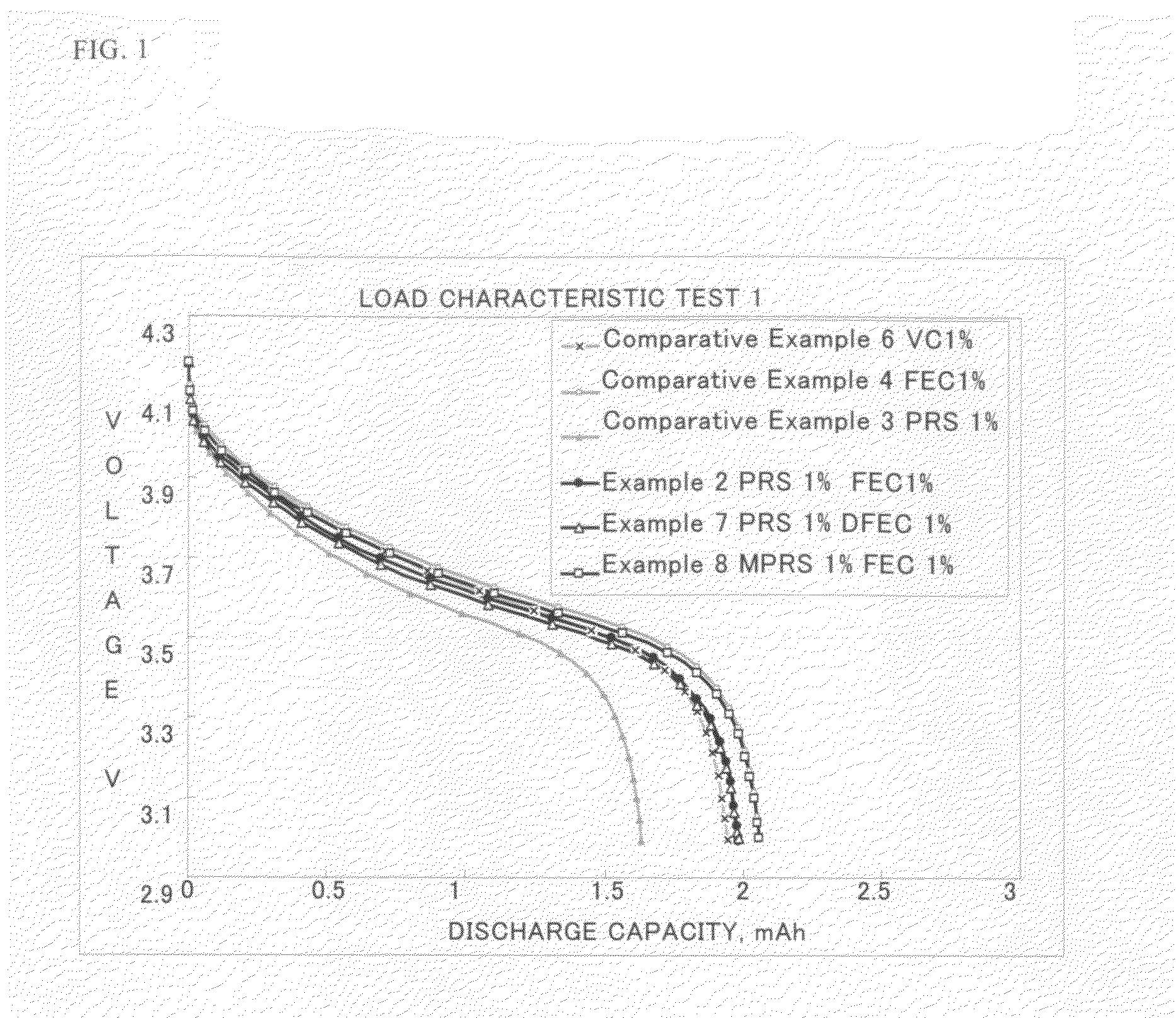
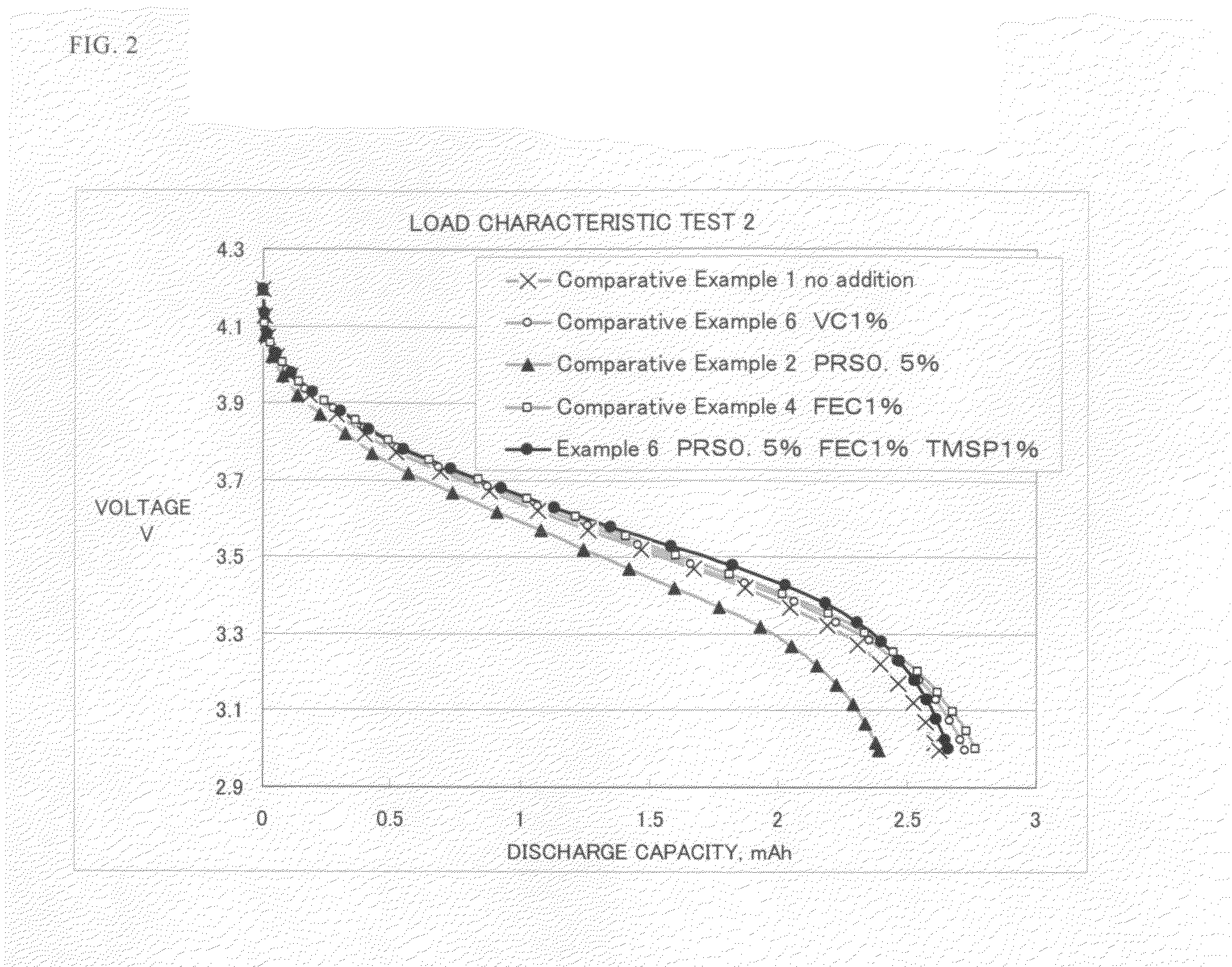
A non-aqueous electrolyte containing propylene carbonate and 1,3-propanesultone as additives can reduce the amount of a gas evolved during storage at a high temperature of a non-aqueous electrolyte secondary cell comprising the electrolyte, a non-aqueous electrolyte containing at least one compound selected from the group consisting of vinylene carbonate, diphenyl disulfide, di-p-tolyldisulfide and bis(4-methoxyphenyl)disulfide as an additive can improve cycle characteristics of a non-aqueous electrolyte secondary cell comprising the electrolyte, and a non-aqueous electrolyte containing a combination of the above two types of additives can provide a non-aqueous electrolyte secondary cell exhibiting excellent retention of capacity and storage stability.
Owner:PANASONIC CORP +1
Polyolefin microporous membrane and method of evaluating the same
The invention relates to a microporous membrane, which is provided with high safety even under a condition that the interior temperature of a battery becomes high, and which has high permeability and high mechanical strength at the same time. The polyolefin microporous membrane is characterized by a membrane thickness of 5 to 50 μm, a void content of 30 to 60%, a gas transmission rate of 40 to 300 sec / 100 cc / 20 μm, a piercing strength of not less than 2.5 N / 20 μm and a break through temperature of not lower than 110° C. The separator in accordance with the present invention is used to exhibit high safety under a high temperature condition as well as high permeability, and therefore it is particularly useful as a separator for miniaturized high capacity batteries of a non-aqueous electrolytic solution type.
Owner:ASAHI KASEI CHEM CORP
Fluoride ion electrochemical cell
The present invention provides electrochemical cells capable of good electronic performance, particularly high specific energies, useful discharge rate capabilities and good cycle life. Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using anion charge carriers capable of accommodation by positive and negative electrodes comprising anion host materials, which entirely eliminate the need for metallic lithium or dissolved lithium ion in these systems.
Owner:CALIFORNIA INST OF TECH +1
Separator for use in non-aqueous electrolyte secondary battery and non-aqueous electrolyte secondary battery
ActiveUS20090148762A1Preventing reduction in output performanceBattery safetyNegative electrodesWound/folded electrode electrodesPorous layerAramides
A separator for use in a non-aqueous electrolyte secondary battery including a first porous layer (layer A) having a shutdown function which becomes substantially a non-porous layer at a high temperature, and a second porous layer (layer B) including an aramid resin and an inorganic material, wherein a ratio (TA / TB) of a thickness (TA) of the layer A relative to a thickness (TB) of the layer B is 2.5 or more and or less.
Owner:PANASONIC CORP +1
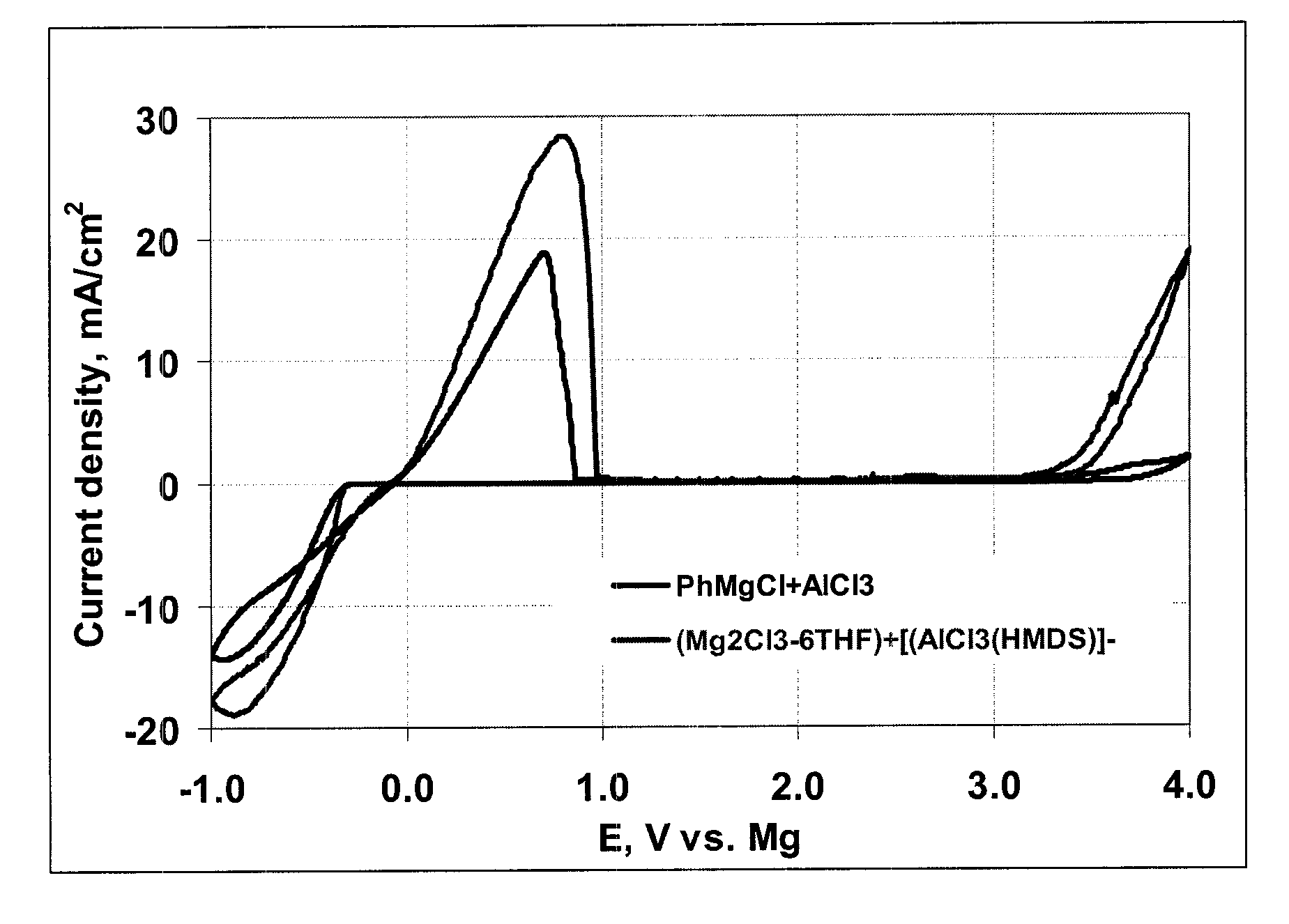
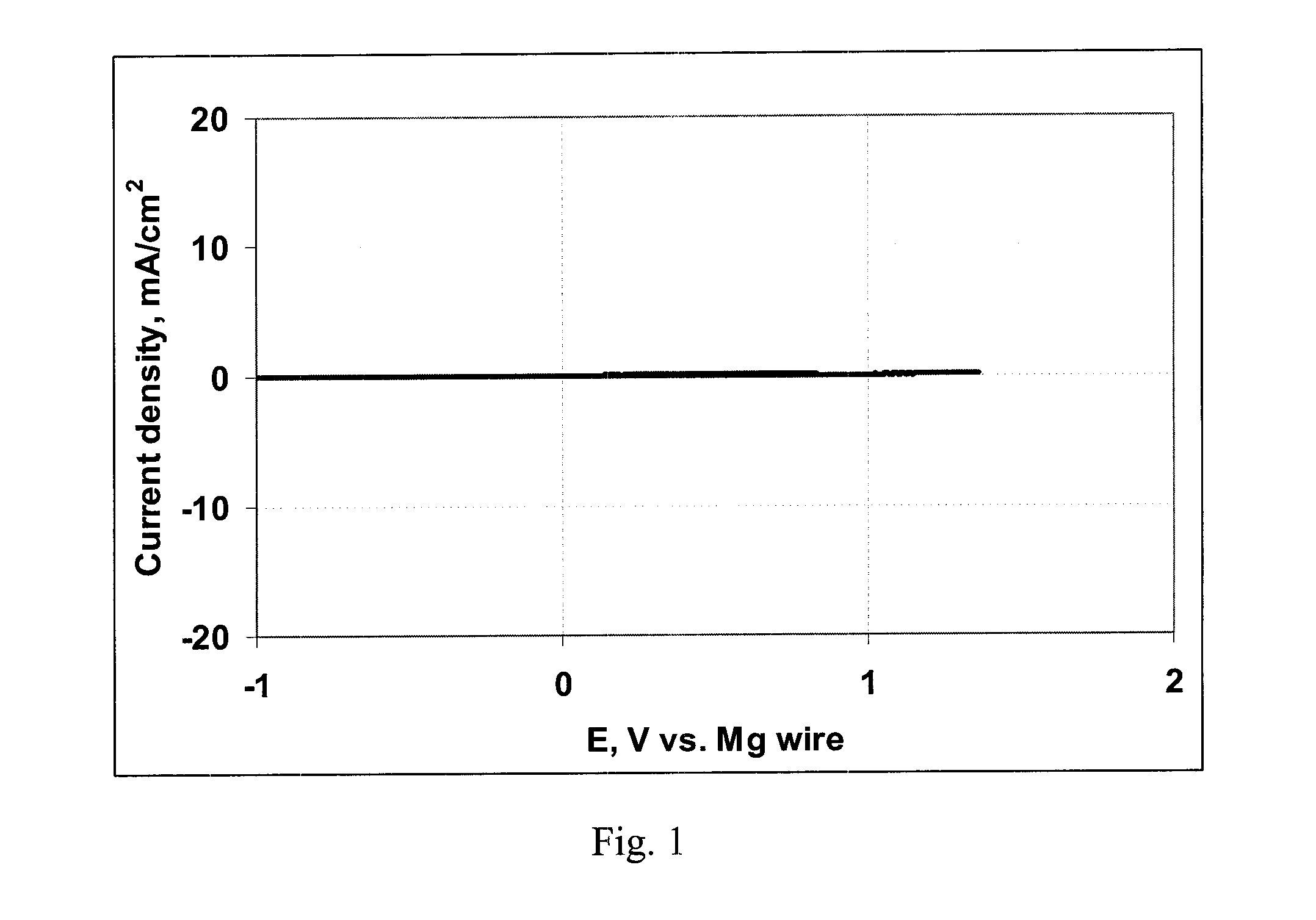
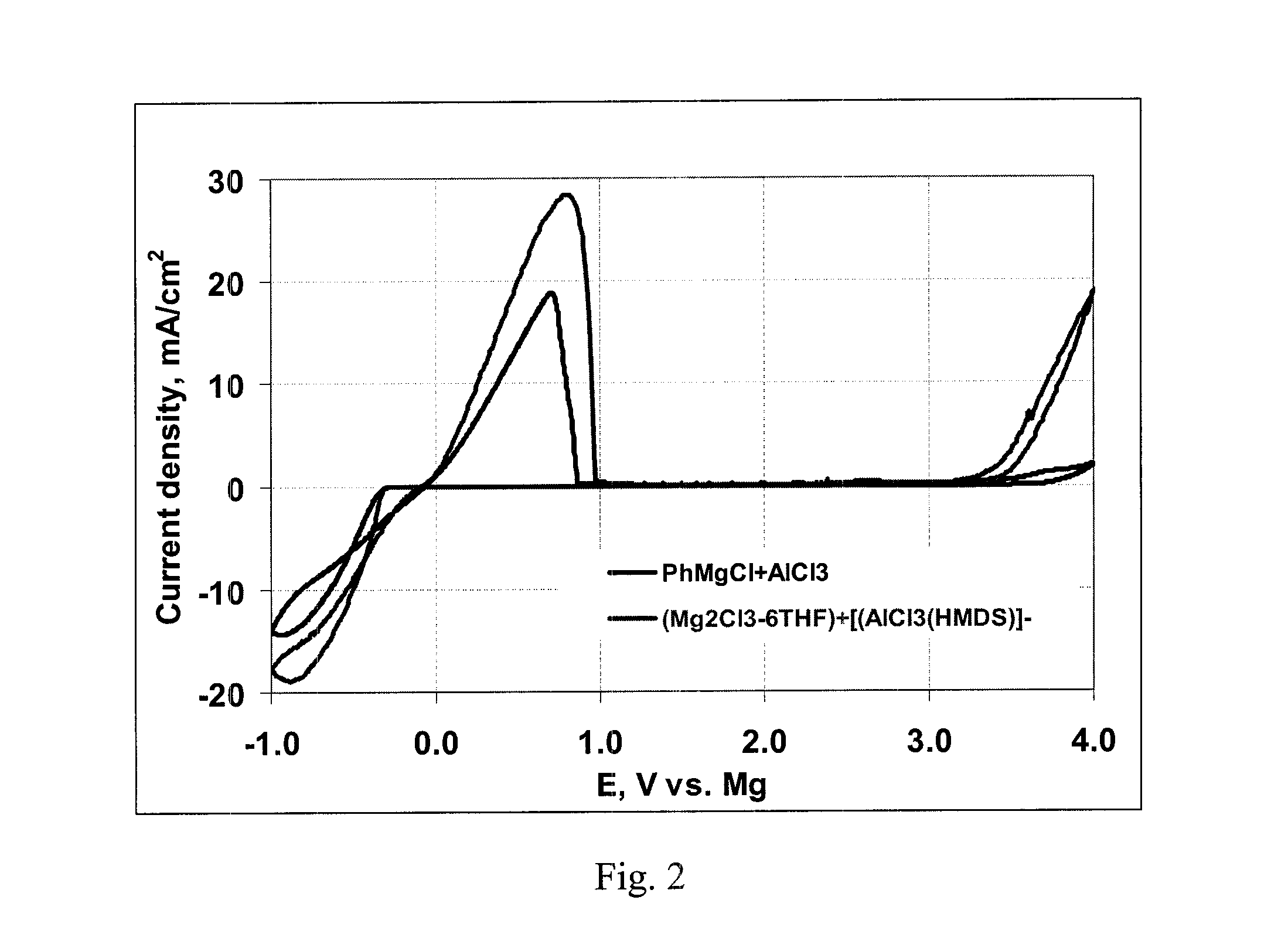
Electrochemical device with a magnesium anode and a stable, safe electrolyte compatible with sulfur
InactiveUS20120107698A1Ease of costManufacturing EaseAlkaline accumulatorsOrganic electrolyte cellsLithium chlorideSulfur
An electrochemical device, having an anode containing magnesium; a cathode stable to a voltage of at least 3.2 V relative to a magnesium reference; and an electrolyte containing a solvent and a LiCl complex of a magnesium halide salt of a sterically hindered secondary amine is provided. In a preferred embodiment the electrolyte contains tetrahydrofuran and 2,2,6,6-tetramethylpiperidinyl-magnesium chloride-lithium chloride complex.
Owner:TOYOTA MOTOR CO LTD
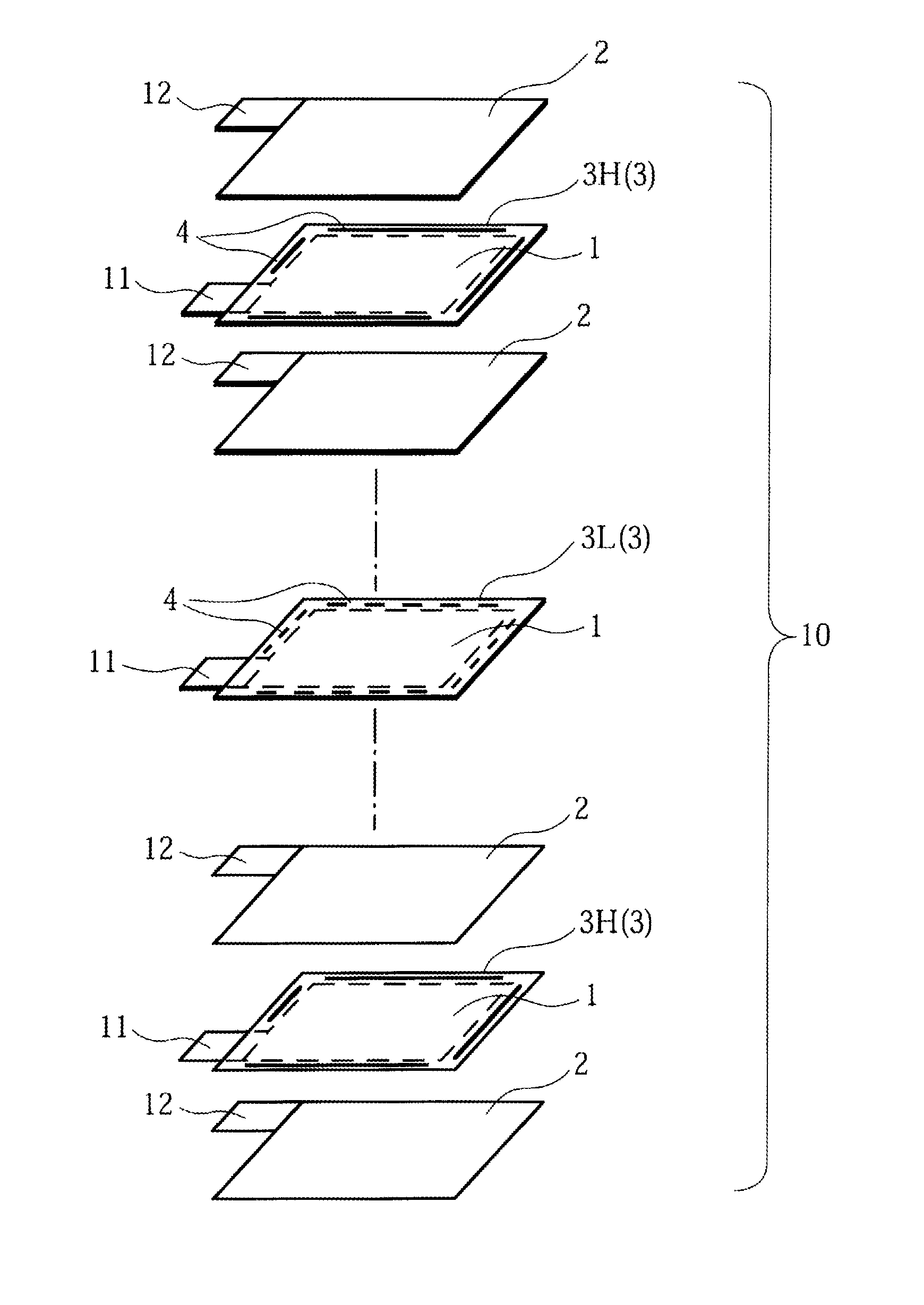
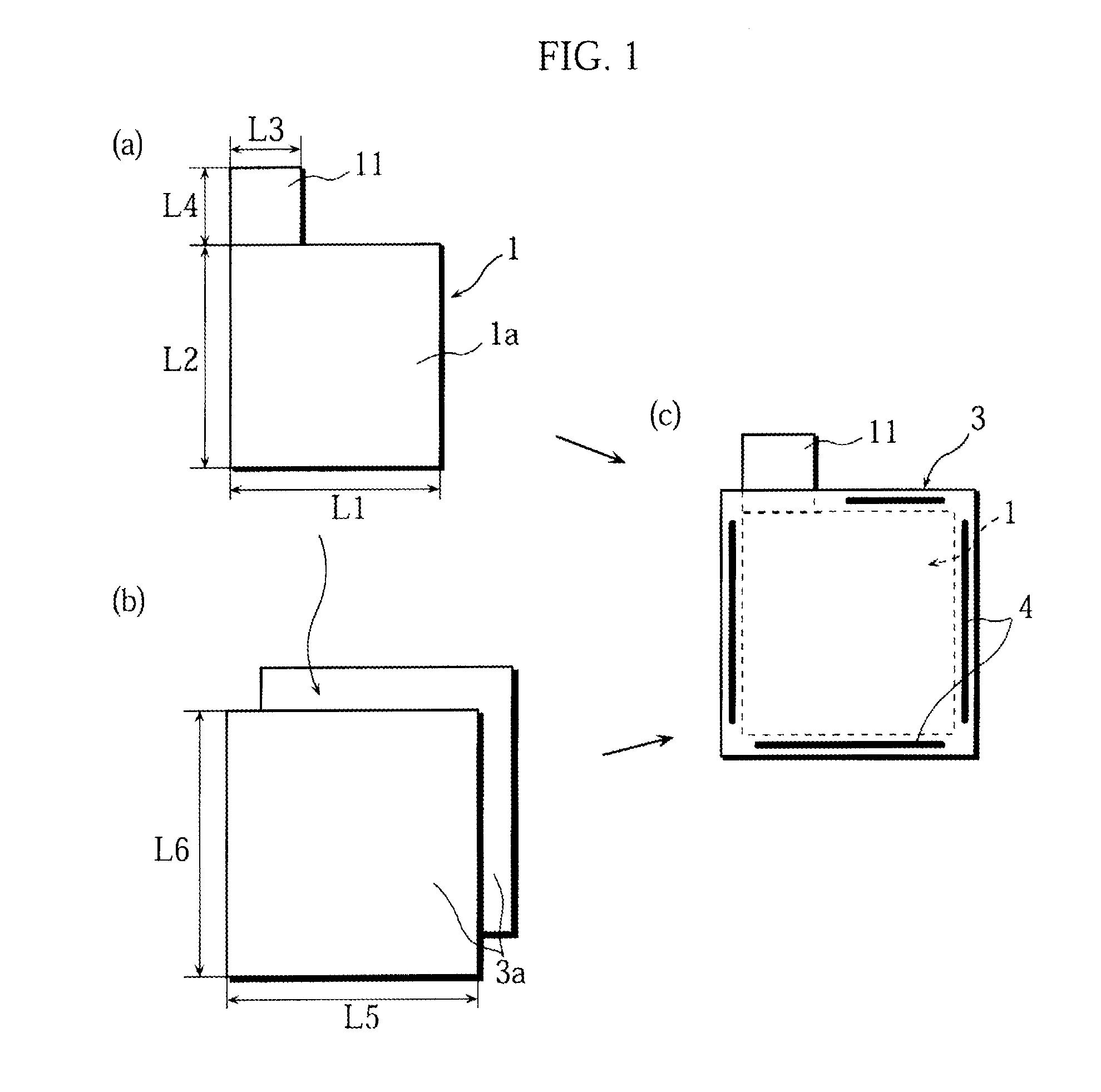
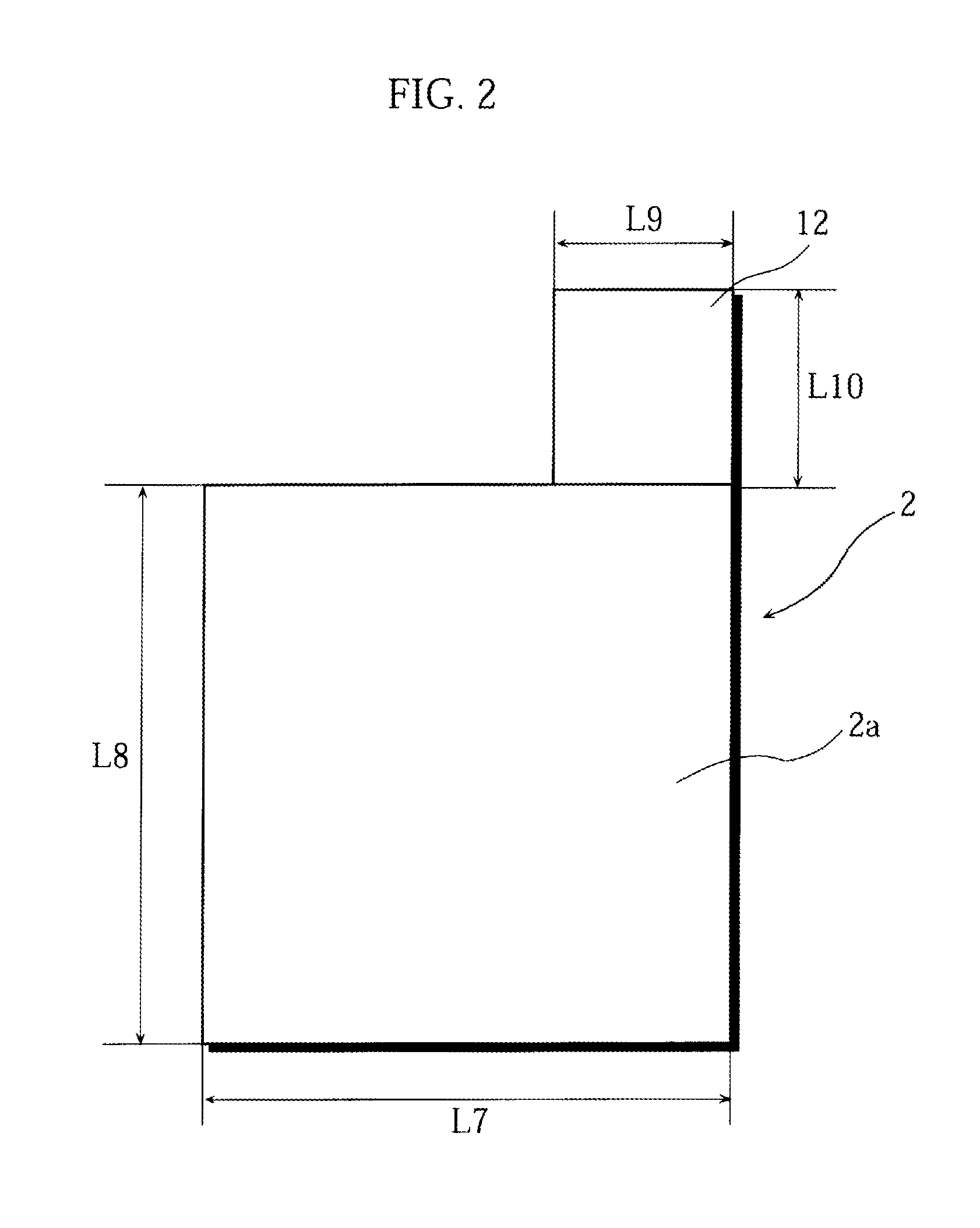
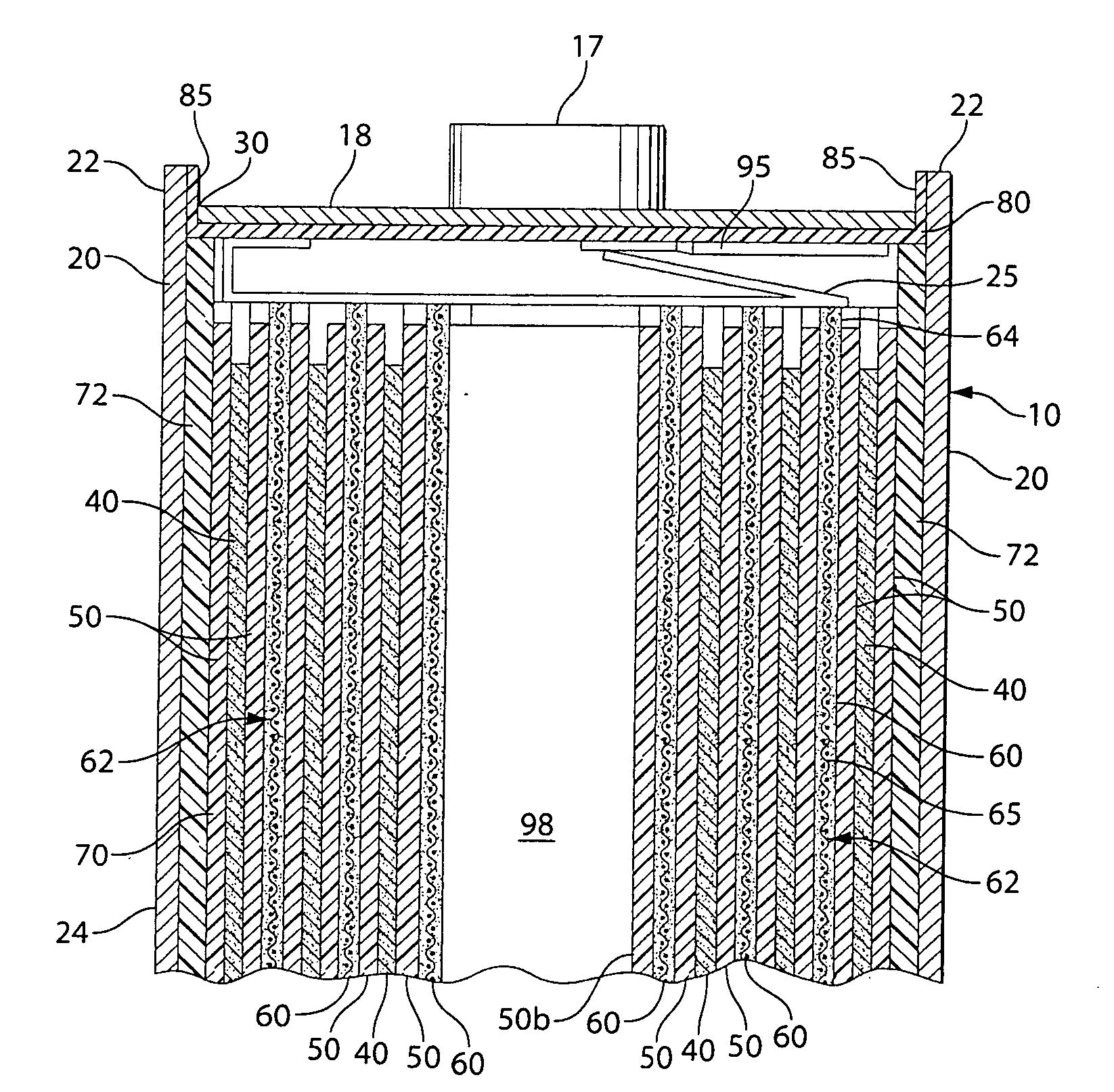
Stack type battery
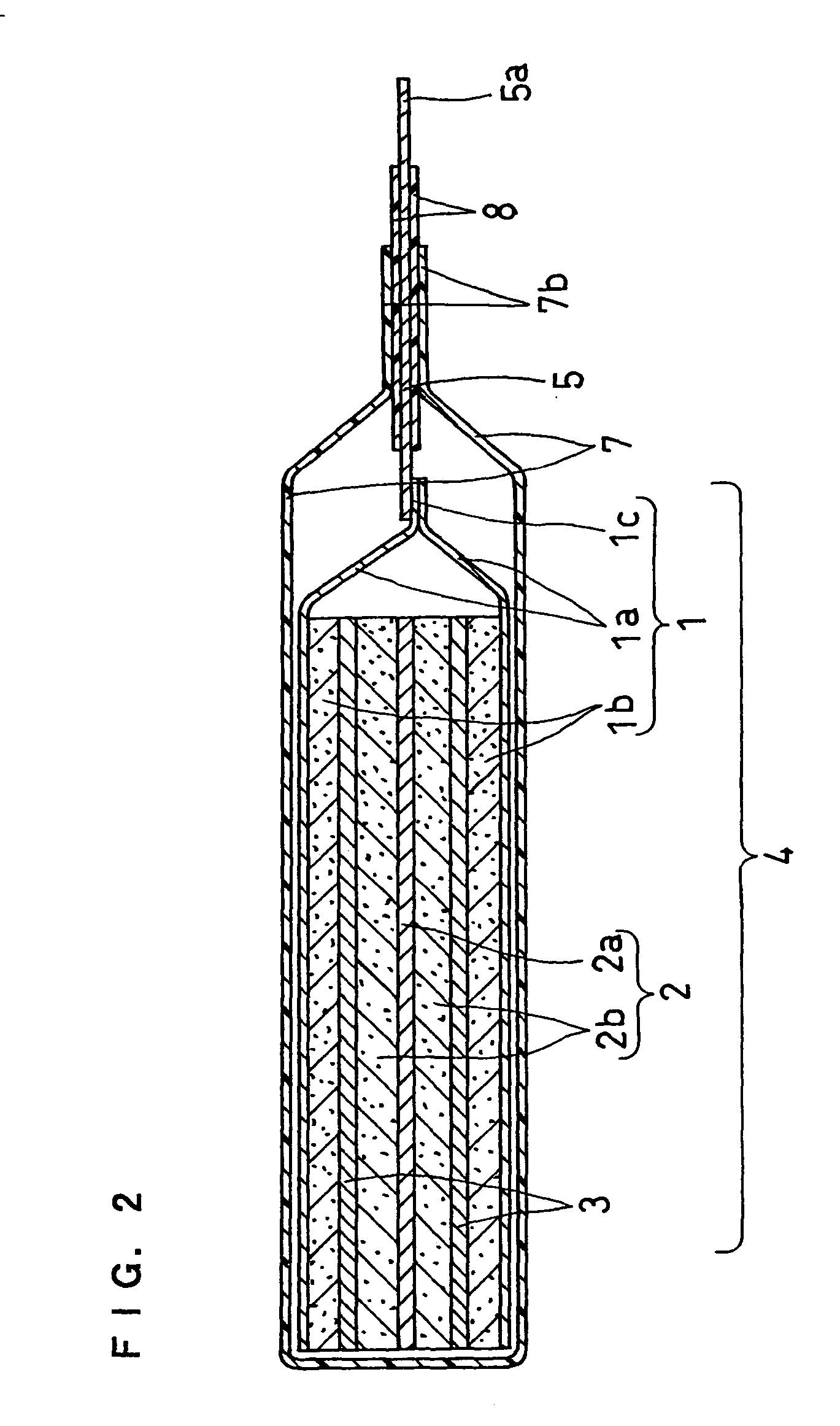
InactiveUS20120077075A1Easy to placeGood effectCell seperators/membranes/diaphragms/spacersLarge-sized flat cells/batteriesEngineeringElectrical and Electronics engineering
A stack type battery has a stacked electrode assembly (10) in which a plurality of positive electrode plates (1) and a plurality of negative electrode plates (2) are alternately stacked one another across separators. Each one of pairs of the separators adjacent to each other in a stacking direction has a bonded portion (4) in which the separators are bonded to each other in at least a portion of a perimeter portion thereof, so as to form a pouch-type separator (3). The proportion of the bonded portion (4) of one of the pouch-type separators 3 (low blocking rate pouch-type separator (3L)) located in a stacking direction-wise central region of the stacked electrode assembly (10) is made smaller than the proportion of the bonded portion (4) of each of the pouch-type separators 3 (high blocking rate pouch-type separator 3H) located in both stacking direction-wise end portions of the stacked electrode assembly (10).
Owner:SANYO ELECTRIC CO LTD
Separation membrane and lithium-sulfur battery comprising same
ActiveUS20160233475A1Prevent elutionGrowth inhibitionElectrode carriers/collectorsNegative electrodesLithium–sulfur batteryElution
The present application relates to a separation membrane and a lithium-sulfur battery including the same, and the separation membrane according to the present application prevents elution of lithium polysulfide in a cathode and suppresses growth of a lithium dendrite generated in an anode, and thus has an effect that a life-span and safety of the battery are improved.
Owner:LG ENERGY SOLUTION LTD
Lithium ion fluoride electrochemical cell
InactiveUS20140030559A1Improve performanceImprove securityElectrode manufacturing processesElectrode carriers/collectorsCharge carrierHost material
Electrochemical cells of the present invention are versatile and include primary and secondary cells useful for a range of important applications including use in portable electronic devices. Electrochemical cells of the present invention also exhibit enhanced safety and stability relative to conventional state of the art primary lithium batteries and lithium ion secondary batteries. For example, electrochemical cells of the present invention include secondary electrochemical cells using a combination of anion and cation charge carriers capable of accommodation by positive and negative electrodes independently comprising host materials.
Owner:CALIFORNIA INST OF TECH
High energy density charge-discharge lithium battery
InactiveCN102738442ACell seperators/membranes/diaphragms/spacersCell electrodesLithium metalHigh energy
The invention, belonging to the technical field of electrochemistry, particularly discloses a high energy density charge-discharge lithium battery. The lithium battery comprises a separator, a cathode, an anode and an electrolyte, wherein the separator is solid, lithium ions can reversibly pass through the separator, the cathode is made of lithium metal or lithium alloy, the electrolyte of the cathode side comprises common organic electrolyte, polymer electrolyte, ionic liquid electrolyte or a mixture thereof, the anode is made of common cathode material of a lithium ion battery, and the electrolyte of the anode side comprises an aqueous solution containing lithium salt or a hydrogel electrolyte. Compared with the traditional lithium ion battery, the charge-discharge lithium battery disclosed by the invention has higher voltage, and the energy density of the charge-discharge lithium battery is more than 30% higher. The high energy density charge-discharge lithium battery can be used in the fields of electricity storage and release, etc.
Owner:FUDAN UNIV
Microstructured electrode structures
ActiveUS8841030B2High energy storageImprove energy efficiencyMaterial nanotechnologyFuel cells groupingMicrometerEngineering
A structure for use in an energy storage device, the structure comprising a backbone system extending generally perpendicularly from a reference plane, and a population of microstructured anodically active material layers supported by the lateral surfaces of the backbones, each of the microstructured anodically active material layers having a void volume fraction of at least 0.1 and a thickness of at least 1 micrometer.
Owner:ENOVIX CORP
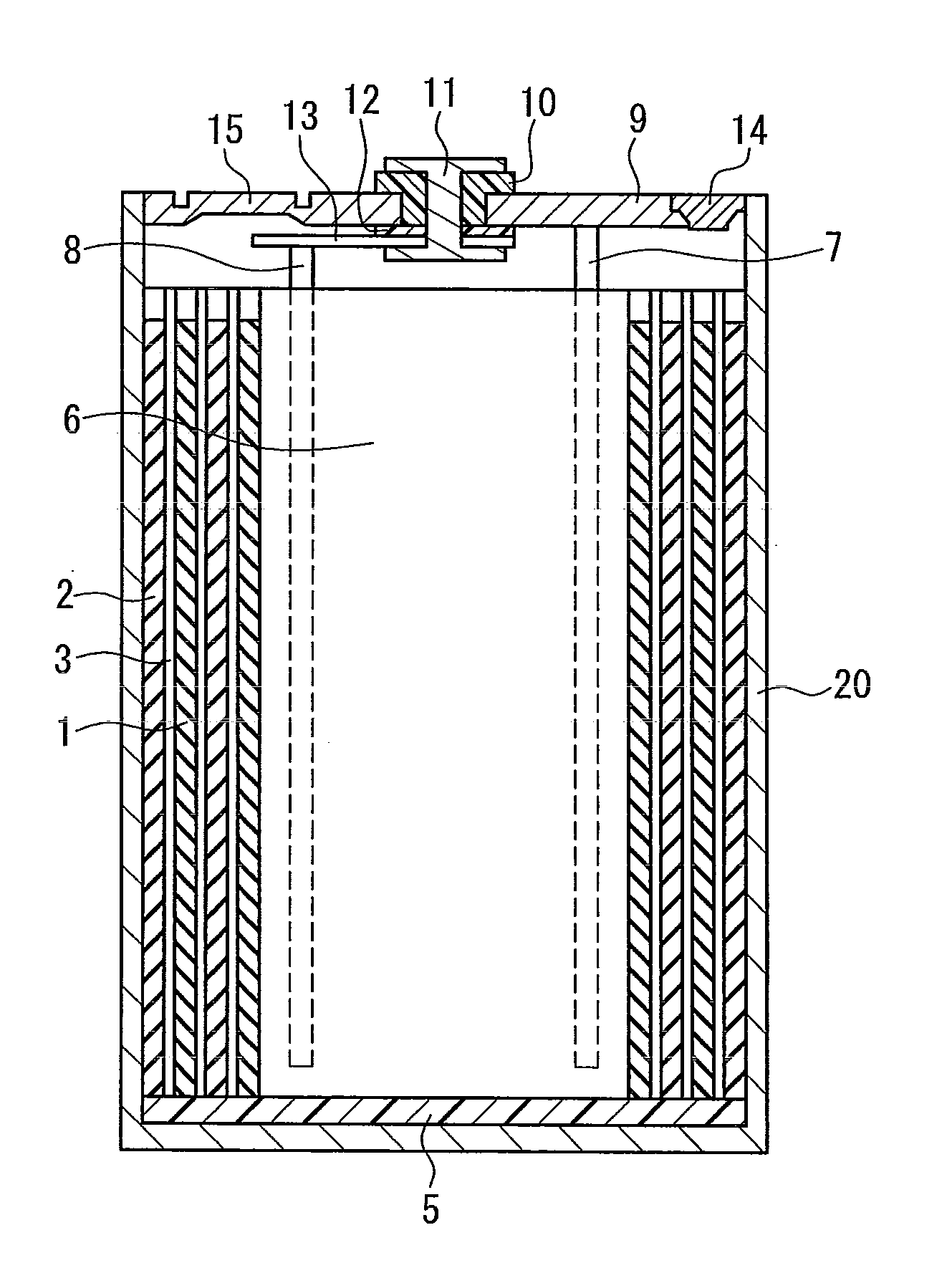
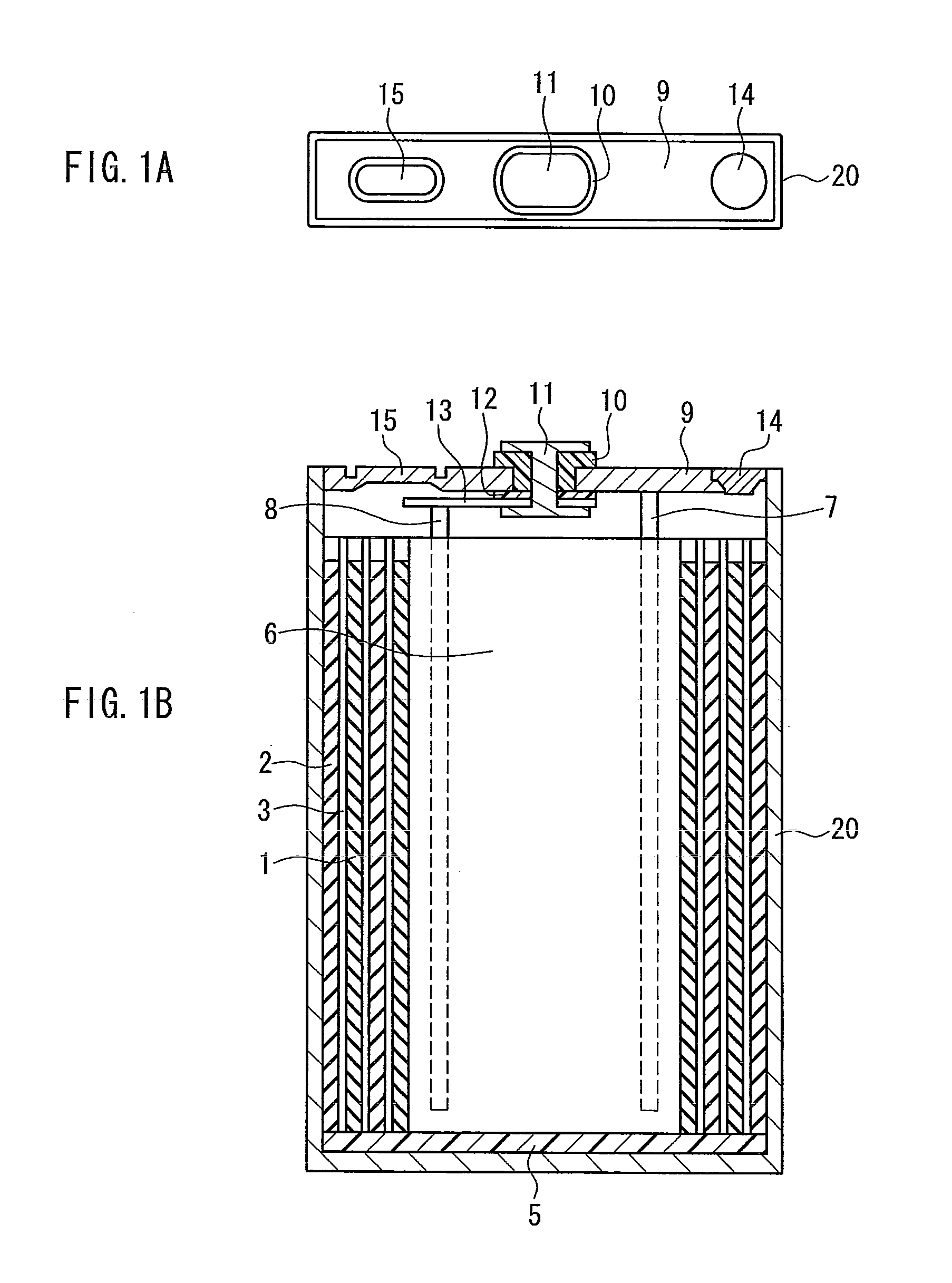
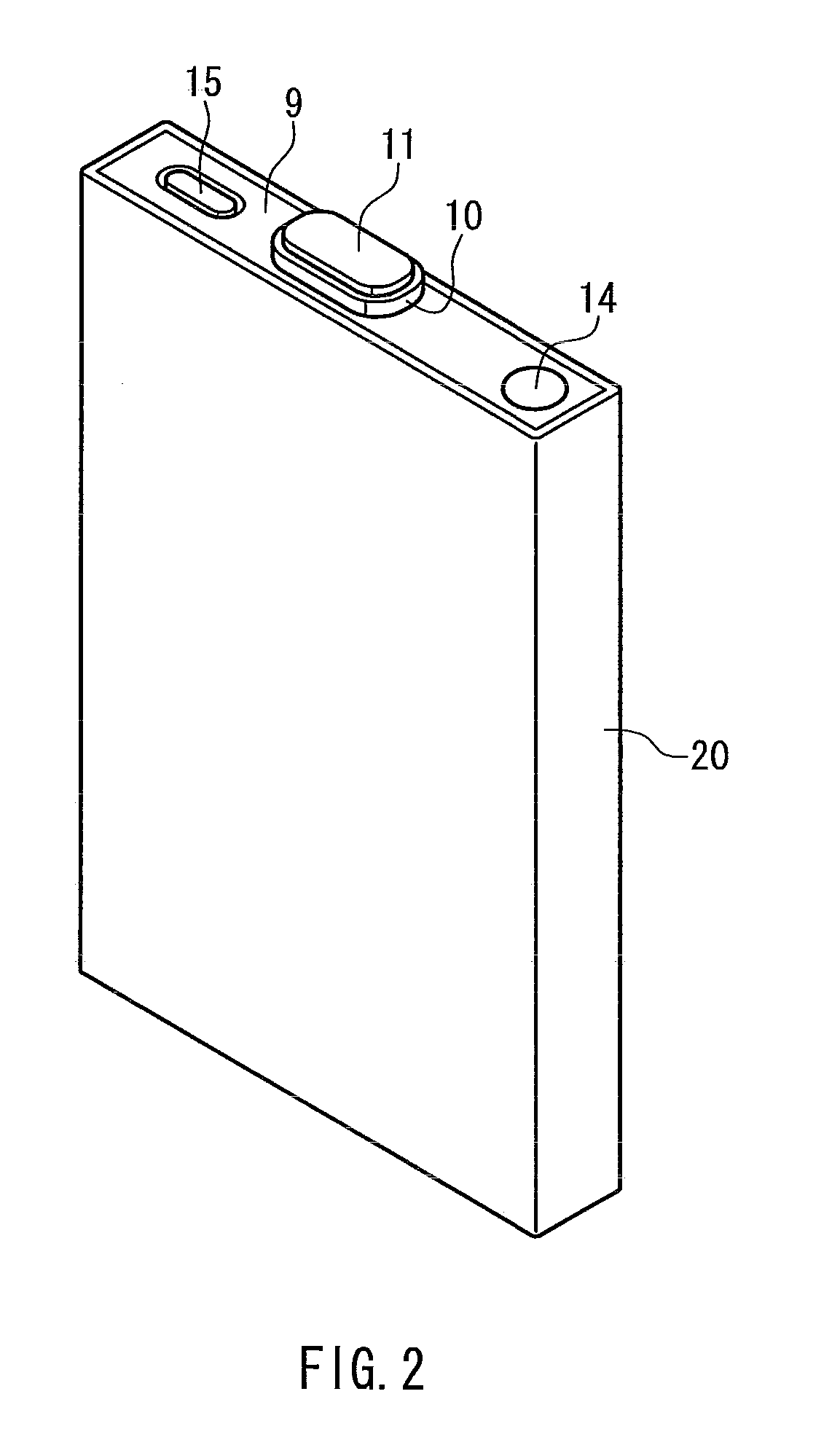
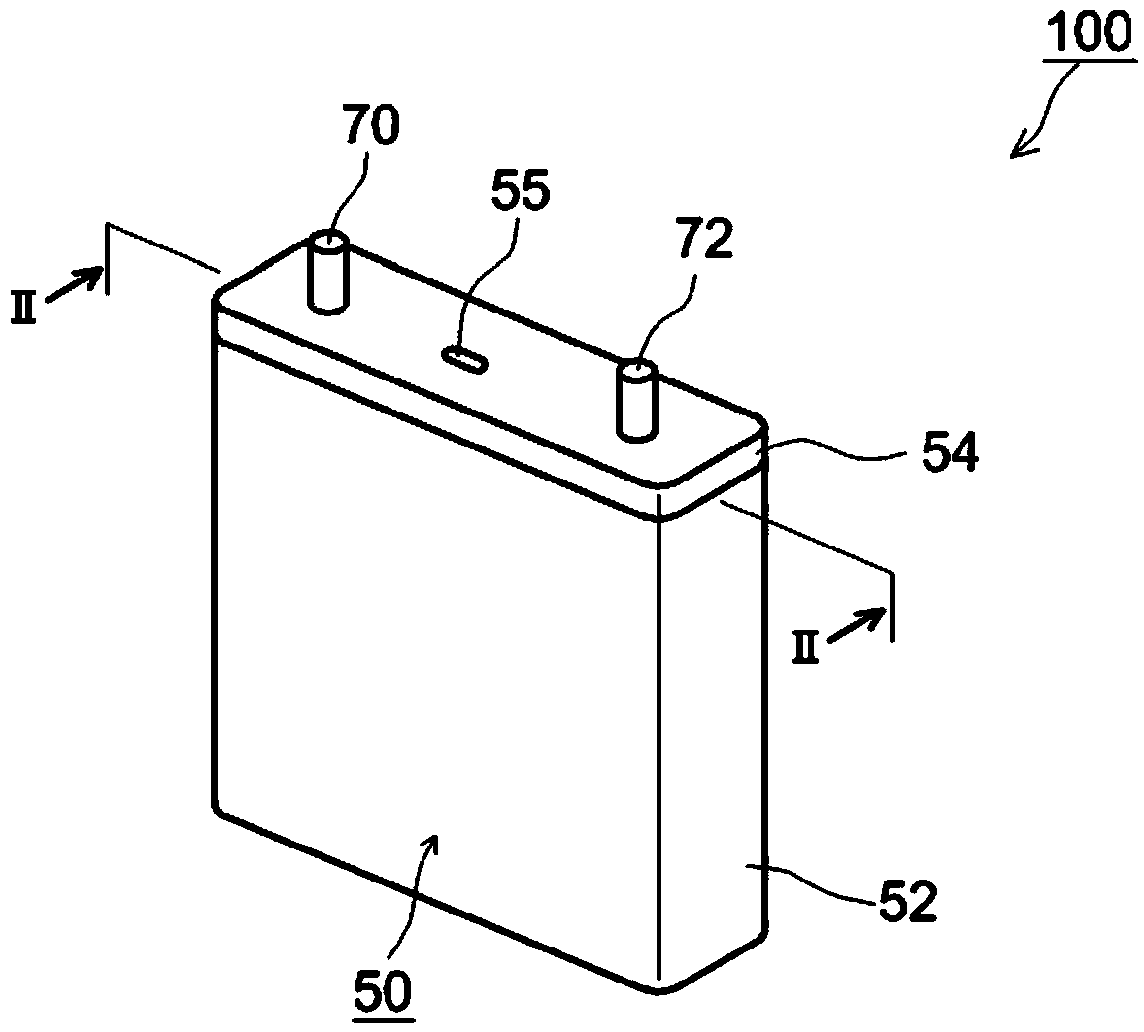
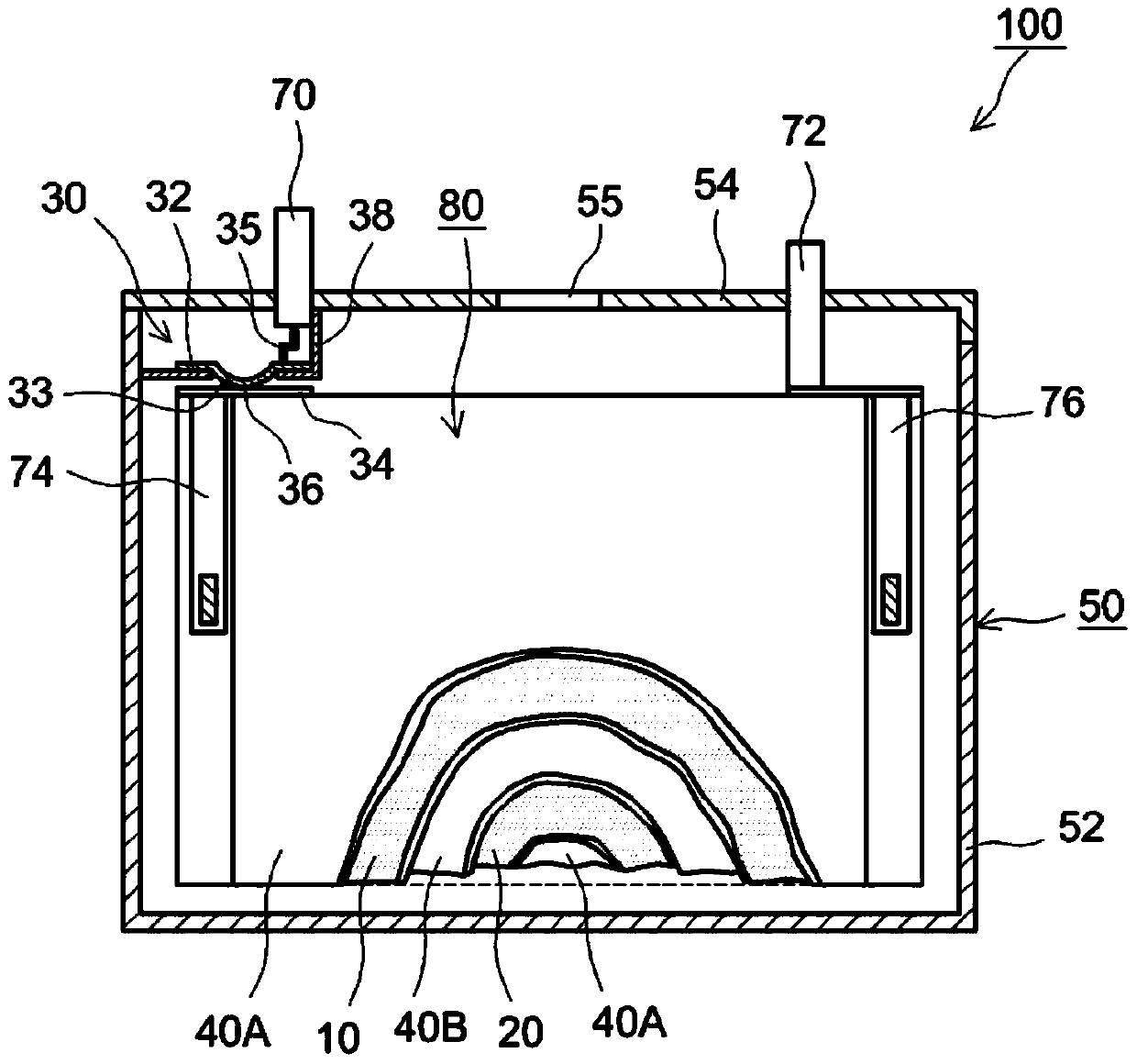
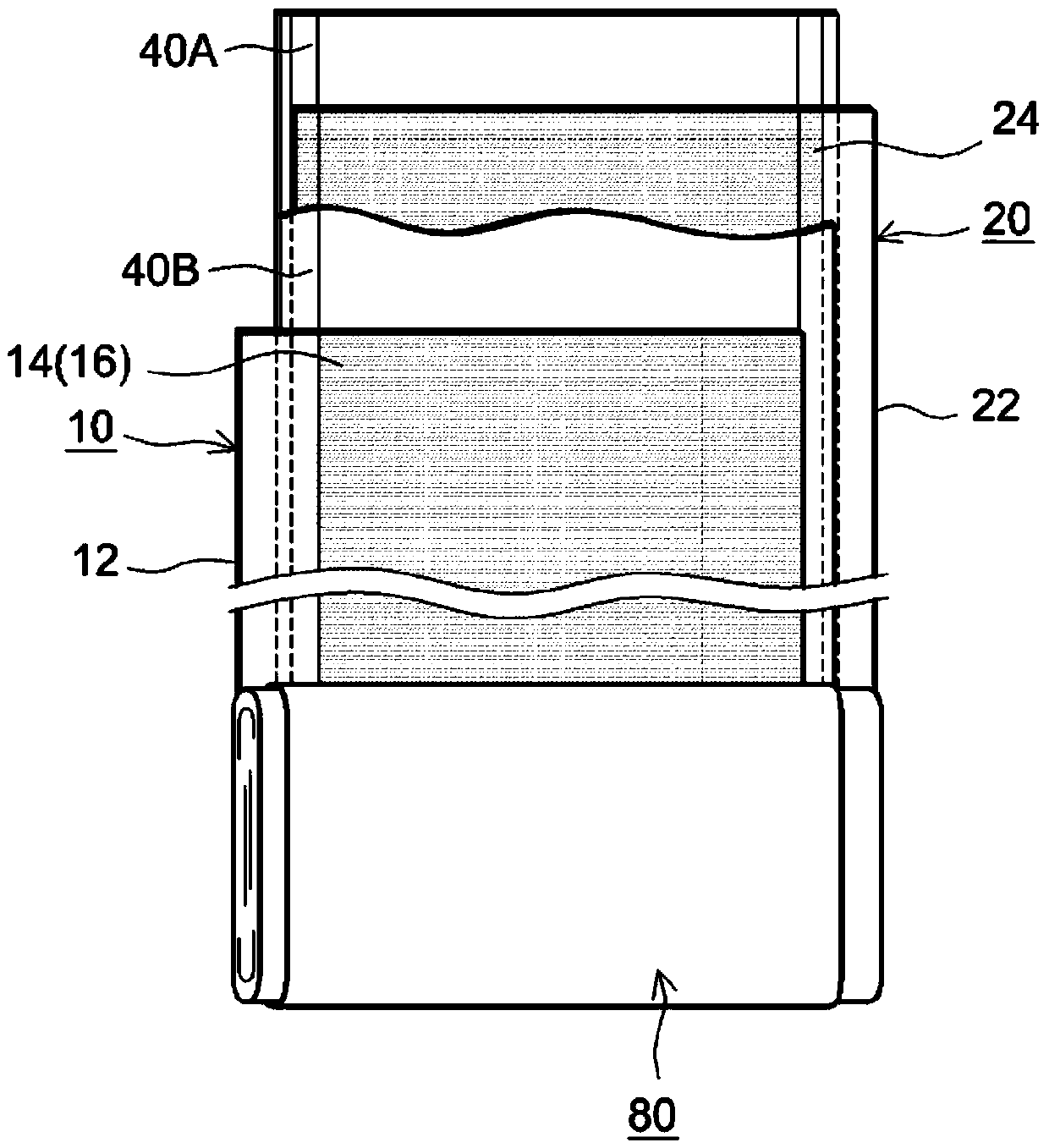
Sealed nonaqueous electrolyte secondary battery
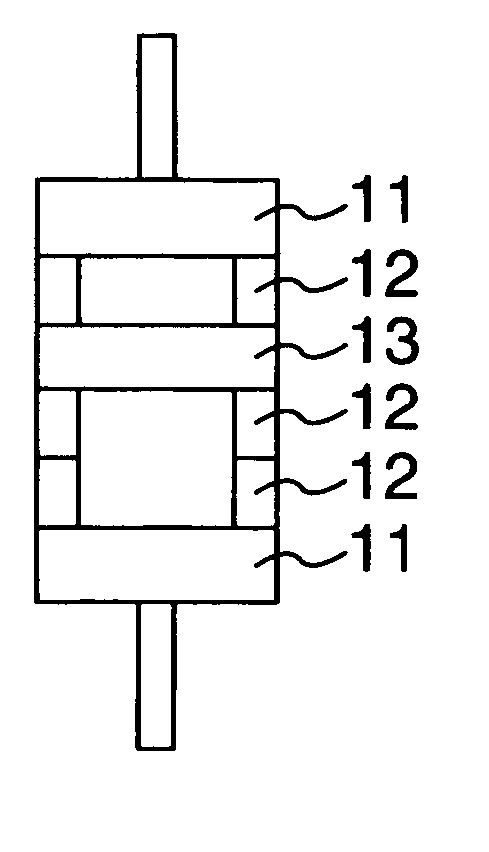
ActiveUS20150010784A1Improve reliabilityImprove battery performanceFinal product manufactureSmall-sized cells cases/jacketsPorosityInternal pressure
The present invention provides a sealed nonaqueous electrolyte secondary battery which is equipped with a current interrupt device that is actuated by a rise in internal pressure of a battery case and in which the current interrupt device is actuated in a speedy and stable manner during an overcharge. In the sealed nonaqueous electrolyte secondary battery, an electrode body formed by a positive electrode 10 and a negative electrode that oppose each other via a separator, an electrolyte, and an overcharge inhibitor are housed in the battery case. The positive electrode 10 includes a positive electrode current collector 12 and a positive electrode active material layer 14 which is formed on the current collector and which mainly contains a positive electrode active material. In addition, a conductive material layer 16 which mainly contains a conductive material is formed between the positive electrode active material layer 14 and the separator. A porosity of the conductive material layer 16 is 35% or more and 55% or less
Owner:TOYOTA JIDOSHA KK
Nonaqueous electrolyte solution and electrochemical element using same
InactiveUS20120171581A1Improve propertiesSilicon organic compoundsOrganic compound preparationAlkoxy groupCarboxylic acid
Disclosed are a nonaqueous electrolytic solution of an electrolyte dissolved in a nonaqueous solvent, which contains a carboxylate represented by the following general formula (I) in an amount of from 0.01 to 10% by mass of the nonaqueous electrolytic solution; and an electrochemical element using it.(In the formula, R1 represents an alkyl group, an alkenyl group, an alkynyl group, a cycloalkyl group, or a cyanoalkyl group; R2 represents a hydrogen atom, an alkoxy group, a formyloxy group, an acyloxy group, an alkoxycarbonyloxy group, an alkanesulfonyloxy group, an arylsulfonyloxy group, an alkylsilyloxy group, a dialkylphosphoryloxy group, an alkoxy(alkyl)phosphoryloxy group, or a dialkoxyphosphoryloxy group; R3 represents a hydrogen atom, —CH2COOR6, or an alkyl group; R4 represents a hydrogen atom or an alkyl group; R5 has the same meaning as R2, or represents a hydrogen atom, an alkyl group, or —CH2COOR7; R6 and R7 each independently represent an alkyl group, an alkenyl group, an alkynyl group, or a cycloalkyl group; X represents —OR8, -A2-C≡Y2, -A2-C(═O)O-A3-C≡Y2, -A2-C(═O)O-A4 or COOR1; R5 is the same as R1; A1 to A3 each independently represent an alkylene group; A4 represents an alkyl group; Y2 represents CH or N; m indicates an integer of from 0 to 4; n indicates 0 or 1.)
Owner:UBE IND LTD
Flat-shaped non-aqueous electrolyte secondary battery
InactiveUS20080070109A1Improve leak-proof effectFinal product manufactureJackets/cases materialsSurface layerEngineering
A flat-shaped non-aqueous electrolyte secondary battery of the present invention includes: an electrode body formed by opposing a positive electrode and a negative electrode while interposing a separator therebetween; an outer case for housing the electrode body; and a sealing plate for sealing an opening of the outer case, and an end part of the sealing plate is positioned inside the outer case. Besides, the sealing plate functions as a positive electrode terminal, the outer case functions as a negative electrode terminal, and a surface layer of the sealing plate in contact with the positive electrode is formed with a metal layer made of aluminum or aluminum alloy.
Owner:HITACHT MAXELL LTD
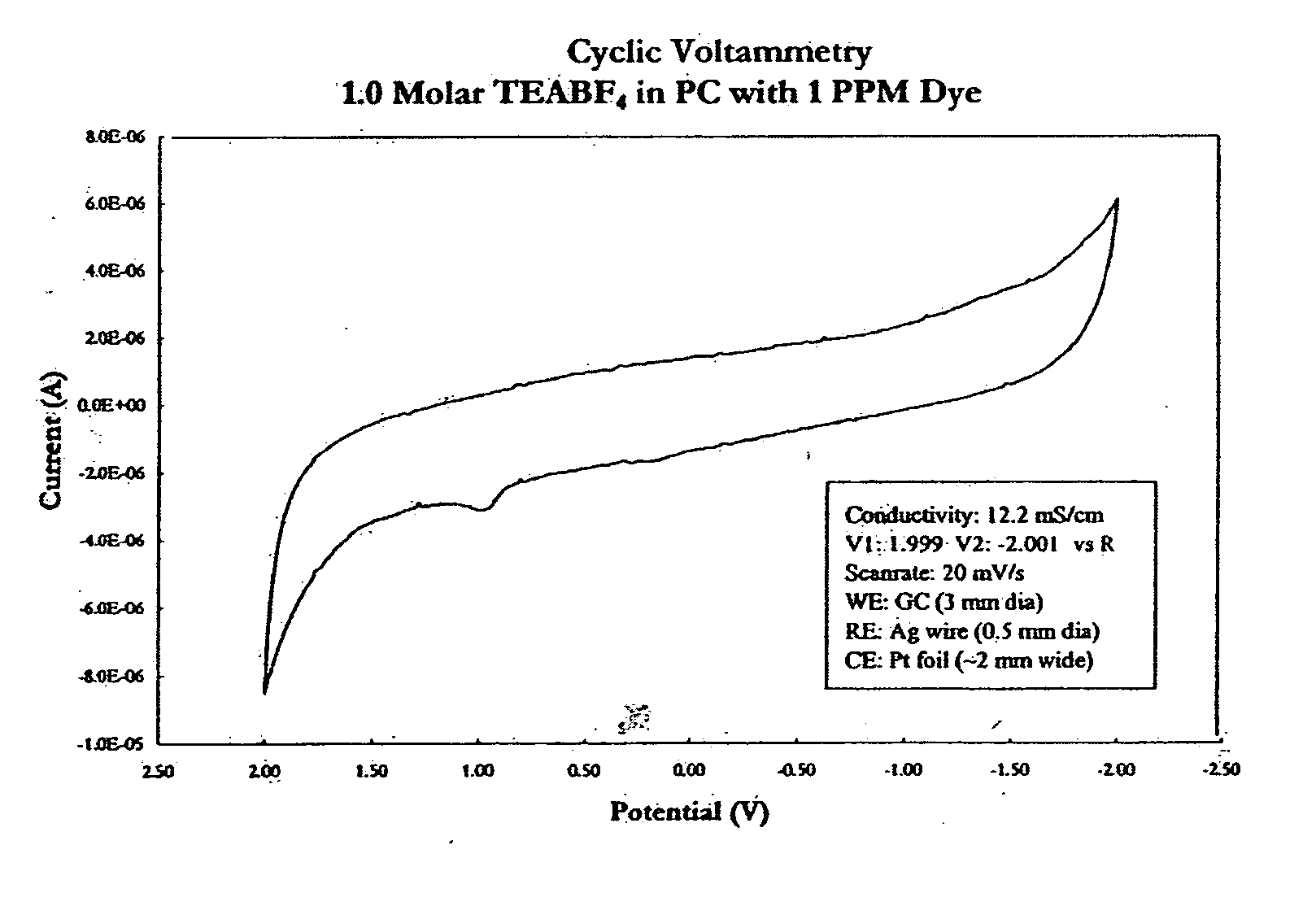
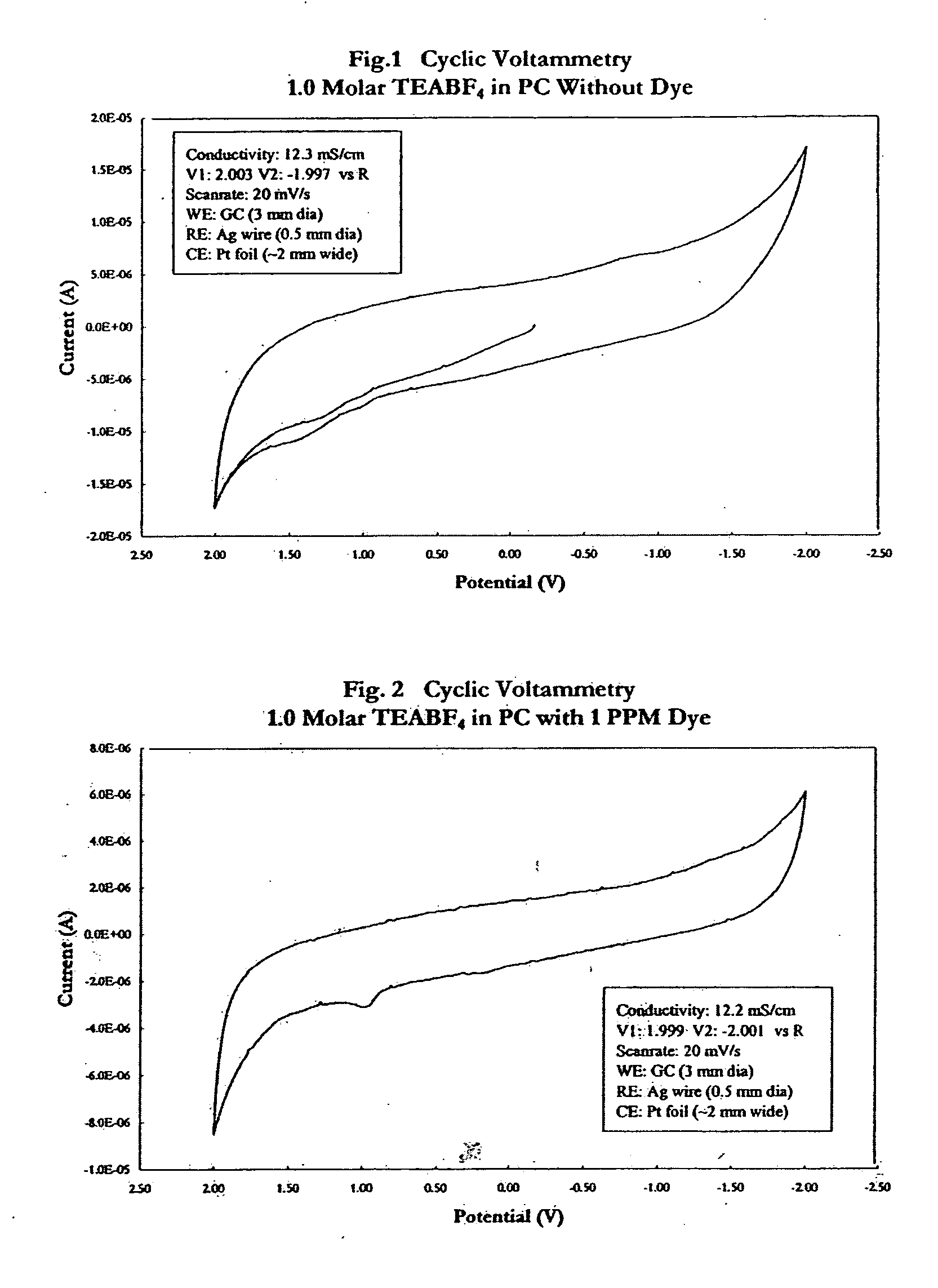
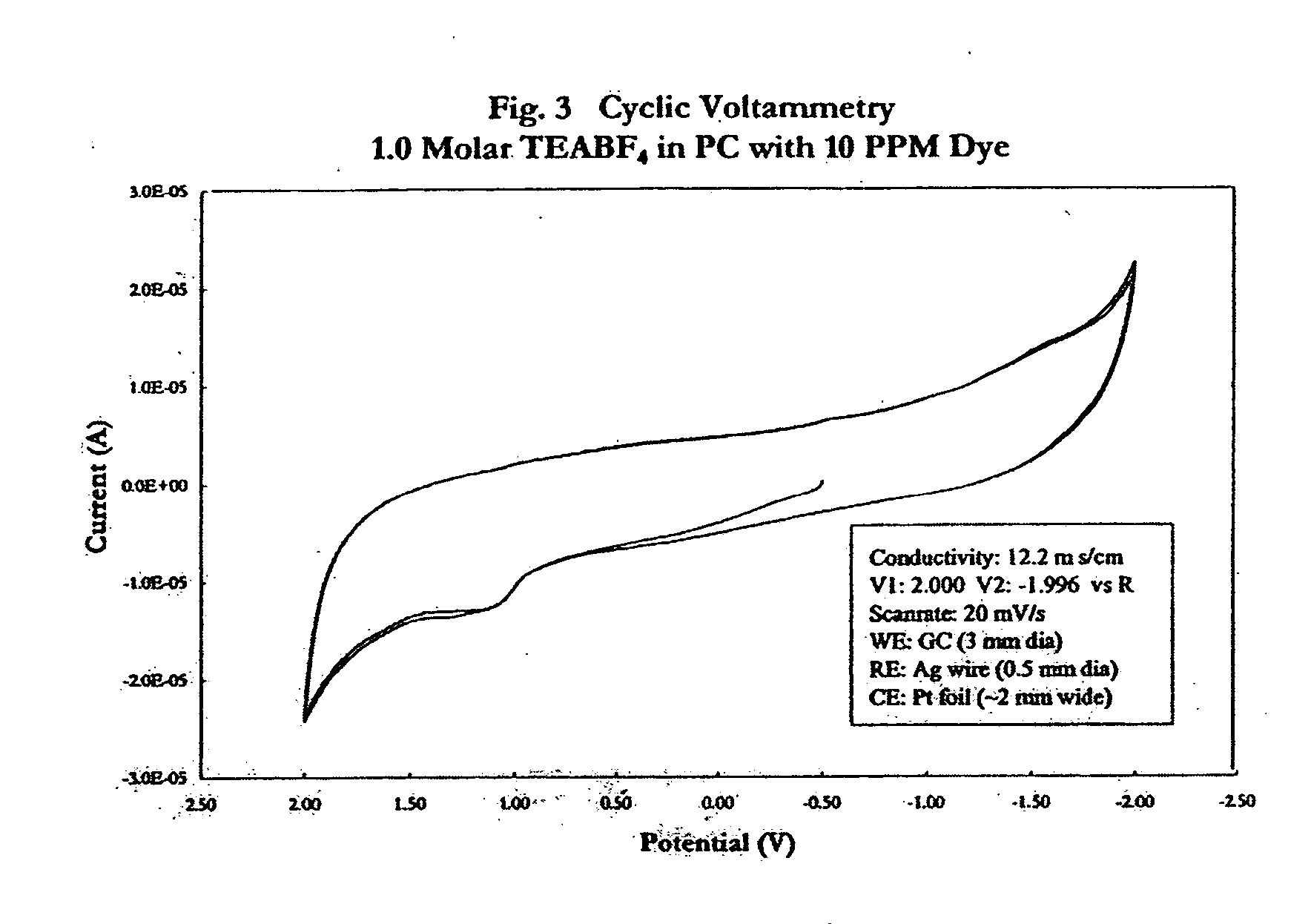
Electrolyte with indicator
InactiveUS20050069761A1Cell/batteries leak testingOrganic electrolyte cellsElectrolyte leakageEngineering
An electrolyte with an indicator, such as a dye, for detecting leakage from an electrochemical energy storage device is provided. Also provided is a method of making such an electrolyte with indicator; a device that incorporates such an electrolyte with indicator; a method of manufacturing an electronic or electrical system that incorporates such a device; and a method of detecting the leakage of electrolyte from a battery or capacitor.
Owner:HONEYWELL INT INC
Negative electrode material for nonaqueous electrolyte secondary battery, making method and lithium ion secondary battery
InactiveCN101847710AHigh 1st cycle charge/discharge efficiencyIncrease capacityMaterial nanotechnologyFinal product manufactureDischarge efficiencyElectrical battery
A negative electrode material comprising composite particles having silicon nano-particles dispersed in silicon oxide is suited for use in nonaqueous electrolyte secondary batteries. The silicon nano-particles have a size of 1-100 nm. The composite particles contain oxygen and silicon in a molar ratio: 0<O / Si<1.0. Using the negative electrode material, a lithium ion secondary battery can be fabricated which features high 1st cycle charge / discharge efficiency, capacity, and cycle performance. The invention also relates to a method for making the negative electrode material.
Owner:SHIN ETSU CHEM CO LTD
Lithium cell with cathode containing iron disulfide
InactiveUS20090317725A1Improve electrochemical performanceLow viscosityOrganic electrolyte cellsPrimary cell electrodesSlurryPrimary cell
A primary cell having an anode comprising lithium and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt dissolved in a solvent mixture which contains 1,3-dioxolane and preferably 1,3-dimethyl-2-imidazolidinone. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:THE GILLETTE CO
Separator and non-aqueous electrolyte secondary battery
ActiveUS20100151325A1Improve cycle performanceEasy to pressNegative electrodesPositive electrodesHeat deflection temperaturePolyolefin
In a non-aqueous electrolyte secondary battery, a separator in which its dynamic hardness DH obtained when the load to an indenter reaches 12 kgf / cm2 is 1000 or more is used. This separator includes at least one porous layer X including a polyolefin, and at least one porous layer Y including a heat resistant resin. The porosity of the porous layer X is 35% or more and 65% or less. In a pore size distribution of the porous layer X measured with a mercury porosimeter, a ratio of pores having a pore size of 0.02 μm or more and 0.2 μm or less is 40 vol % or more relative to a total pore volume. The thermal deformation temperature of the heat resistant resin is 160° C. or more.
Owner:PANASONIC CORP
Nonaqueous electrolyte solution and nonaqueous electrolyte battery employing the same
ActiveUS20150140448A1Improve featuresIncreased durabilityOrganic electrolyte cellsLi-accumulatorsHigh temperature storageElectrolytic agent
Provided is a nonaqueous electrolyte solution battery with dramatically improved battery characteristics. A nonaqueous electrolyte solution battery prepared by using a nonaqueous electrolyte solution comprising (I) an aromatic carboxylic acid ester compound represented by General Formula (1) below; and (II) specific compounds, is improved in charge-discharge characteristics at high current densities, durability performance during high-temperature storage and overcharge characteristics.(in General Formula (1), A1 is —R1 or —OR1, with R1 being an optionally substituted hydrocarbon group with 10 or fewer carbon atoms; A2 is an optionally substituted aryl group; each of j and k is independently 0 or an integer greater than 0, and at least one of j and k is an integer not less than 1; and when k≧1, A1 is —OR1, while when k=0, A1 is —R1; and the case of j=1, k=0 is not allowed).
Owner:MU IONIC SOLUTIONS CORP +1
Lithium ion battery
InactiveUS20120021297A1High glass transition temperatureMaterial nanotechnologyCell seperators/membranes/diaphragms/spacersMechanical stabilitySodium-ion battery
A lithium battery comprising an anode and a cathode structure separated from one another by a membrane structure. The membrane structure comprises a layer which is only conductive to lithium ions and which is characterized by the property of having sufficient mechanical stability at temperatures higher than 150° C. to prevent a local short circuit between the anode and the cathode structure.
Owner:DRITTE PATENTPORTFOLIO BET GMBH & CO KG
Separator for non-aqueous batteries, non-aqueous battery using same, and production method for separator for non-aqueous batteries
InactiveCN102498592ALower initial resistanceImprove output efficiencyNon-aqueous electrolyte accumulatorsCell seperators/membranes/diaphragms/spacersPolymer scienceElectrical battery
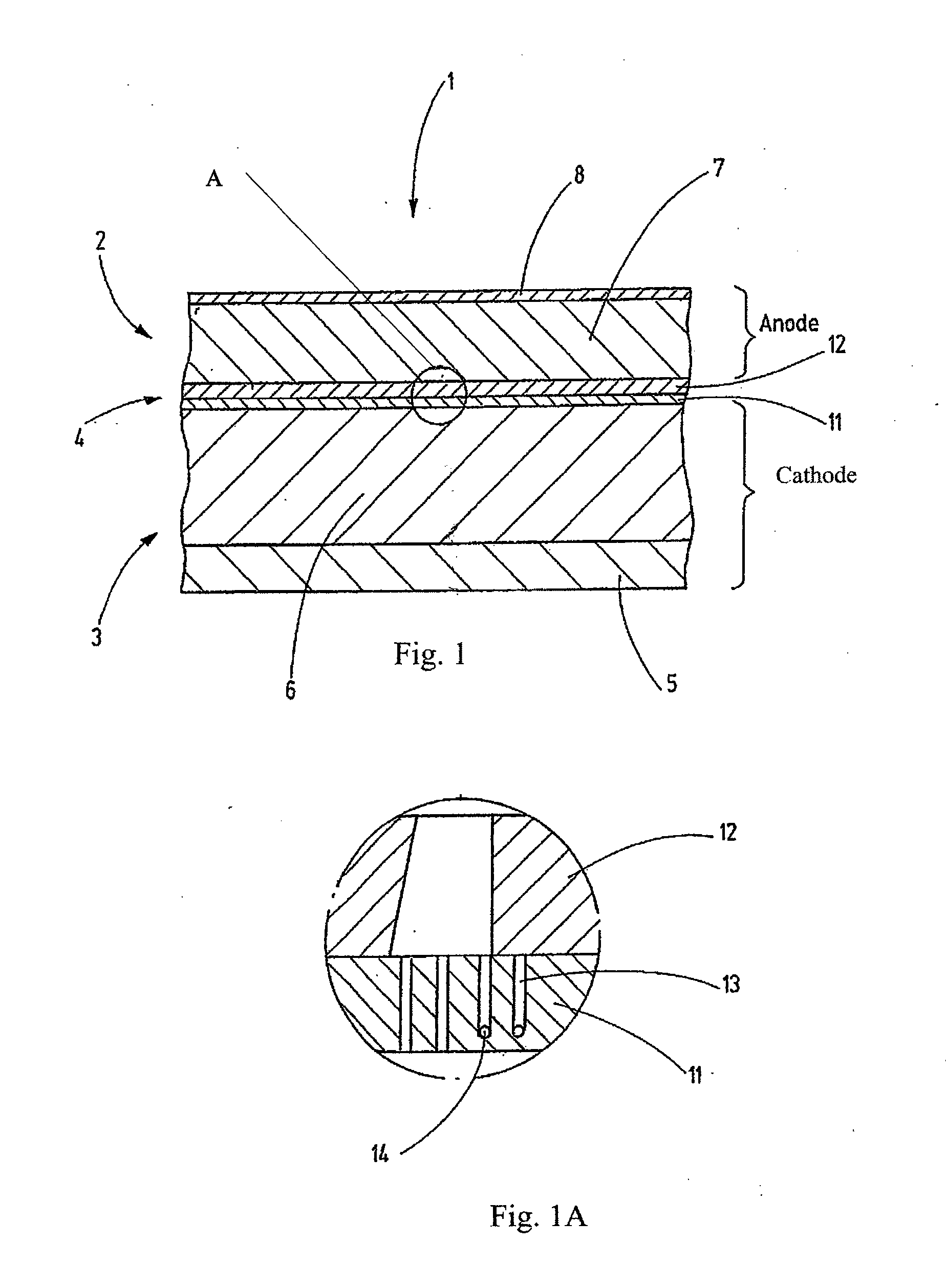
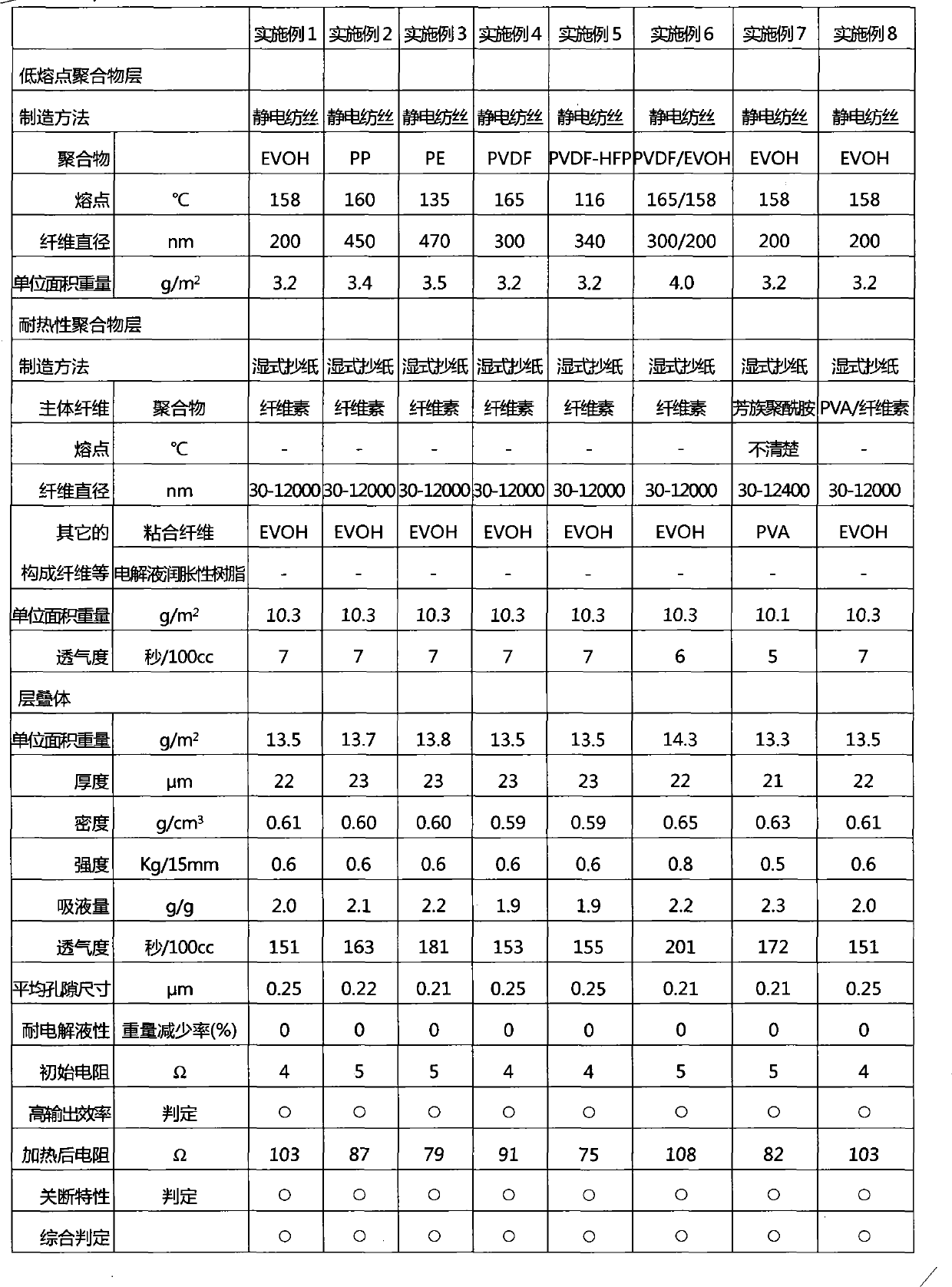
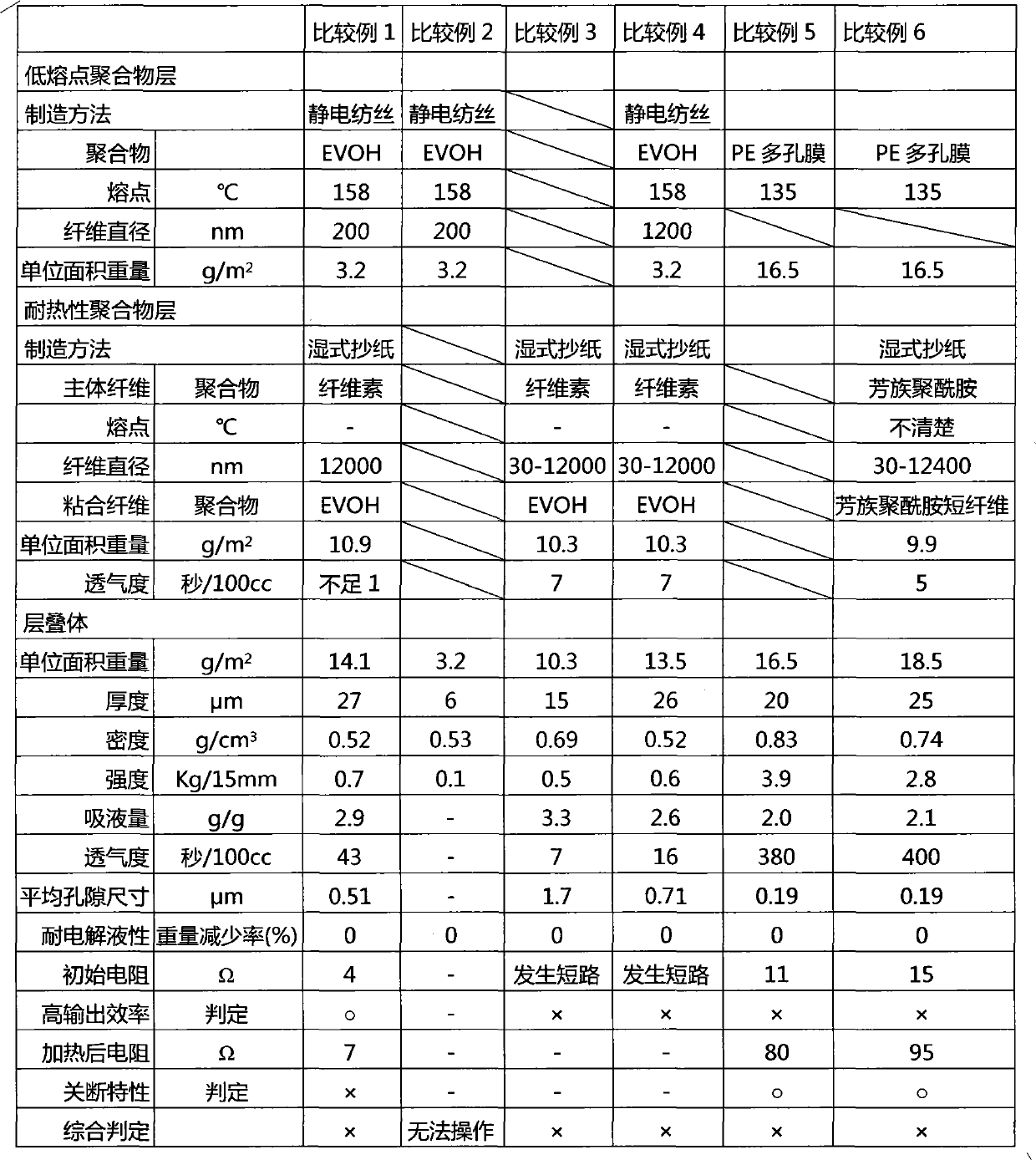
Disclosed is a separator for non-aqueous batteries which not only has a shutdown mechanism, but can also achieve a higher output and has short-circuit resistance. The separator is configured of a laminate which is provided with a low melting point polymer-fibre layer (A), formed of a low melting point polymer having a melting point of 100-200 DEG C, and a heat-resistant polymer-fibre layer (B), formed above the low melting point polymer-fibre layer (A) and formed of a heat infusible polymer or a high melting point polymer, having a melting point of over 200 DEG C. The low melting point polymer-fibre layer (A) includes low melting point polymer fibres having a fibre diameter not greater than 1000nm; the heat-resistant polymer-fibre layer (B) is formed from the heat resistant polymers and includes an amalgam of nanofibres and non-nanofibres.
Owner:KURARAY CO LTD
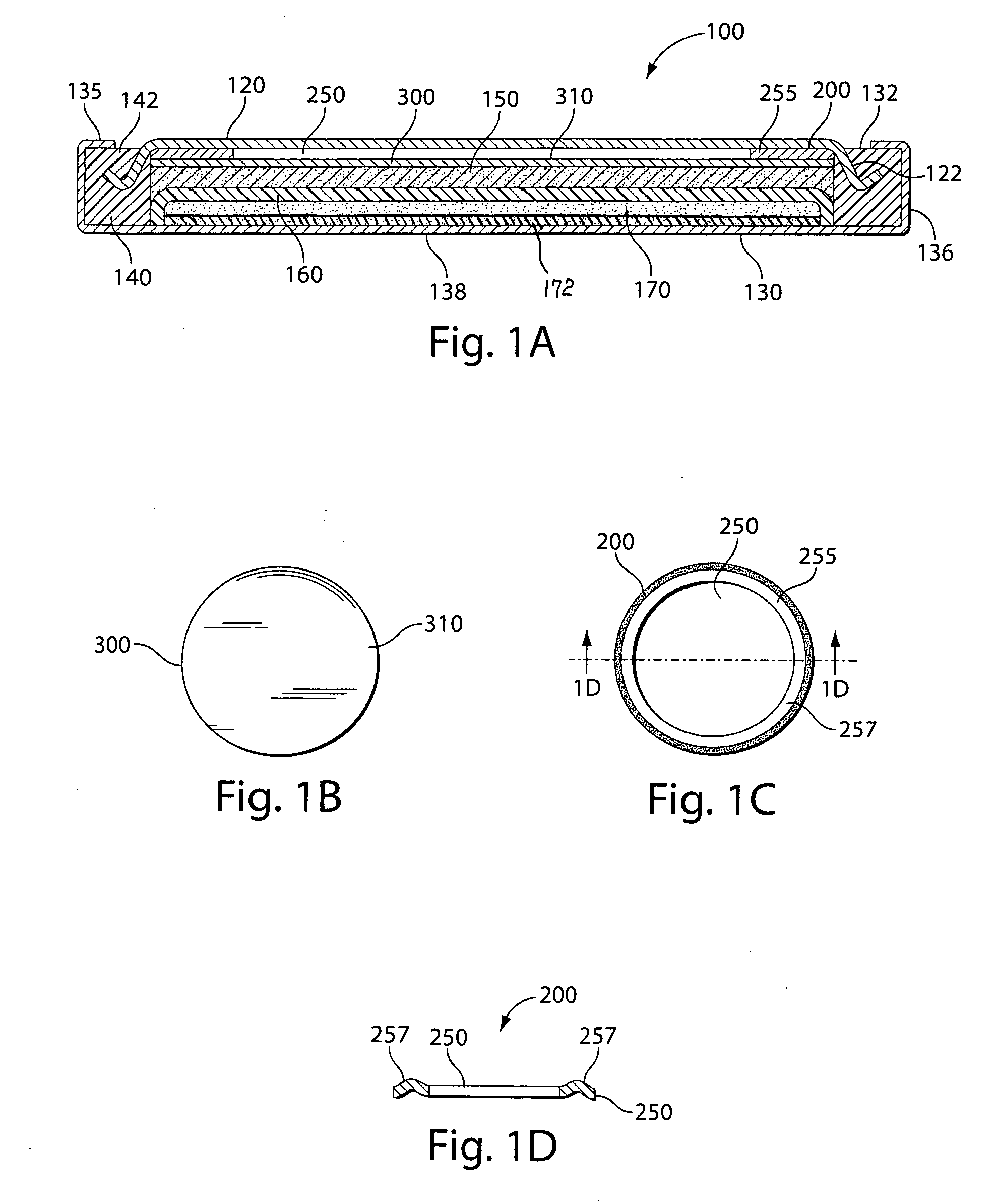
Method of preparing separator for lithium secondary battery, separator prepared therefrom, and lithium secondary battery comprising the same
ActiveUS20150263324A1Easy to handleEasy to storeCell seperators/membranes/diaphragms/spacersFinal product manufactureLithiumPolymer science
The present disclosure provides a method of preparing a separator for a lithium secondary battery, comprising: (S1) bringing polymer particles into electric charging to obtain electrically charged polymer particles; (S2) transferring the electrically charged polymer particles on at least one surface of a porous polymer substrate to form an electrode-adhesion layer whose area ranges from 1 to 30% based on the total area of the porous polymer substrate; and (S3) fixing the electrode-adhesion layer with heat and pressure. In accordance with the present disclosure, an electrode-adhesion layer is applied by using electrostatic charging, more specifically coating polymer particles by way of laser printing, without the addition of a slurry in a solvent, thereby allowing easy handling and storage and needs no drying step of the solvent to provide cost savings effect as well as rapid and efficient preparation of the separator.
Owner:LG ENERGY SOLUTION LTD
Sealed nonaqueous electrolyte secondary battery
ActiveCN104137306AImprove reliabilityImprove battery performanceFinal product manufactureSmall-sized cells cases/jacketsInternal pressurePorosity
Provided is a sealed nonaqueous electrolyte secondary battery, which is equipped with a current blocking mechanism that is actuated by an increase in the internal pressure of a battery case, and wherein the mechanism quickly and stably operates at the time of overcharging. This sealed nonaqueous electrolyte secondary battery is obtained by having a battery case contain an electrode body, in which a positive electrode (10) and a negative electrode face each other with a separator interposed therebetween, an electrolyte and an overcharge inhibitor. The positive electrode (10) is provided with a positive electrode collector (12) and a positive electrode active material layer (14) that is formed on the collector and contains a positive electrode active material as a main component. In addition, a conductive material layer (16), which contains a conductive material as a main component, is formed between the positive electrode active material layer (14) and the separator, and the conductive material layer (16) has a porosity of from 35% to 55% (inclusive).
Owner:TOYOTA JIDOSHA KK
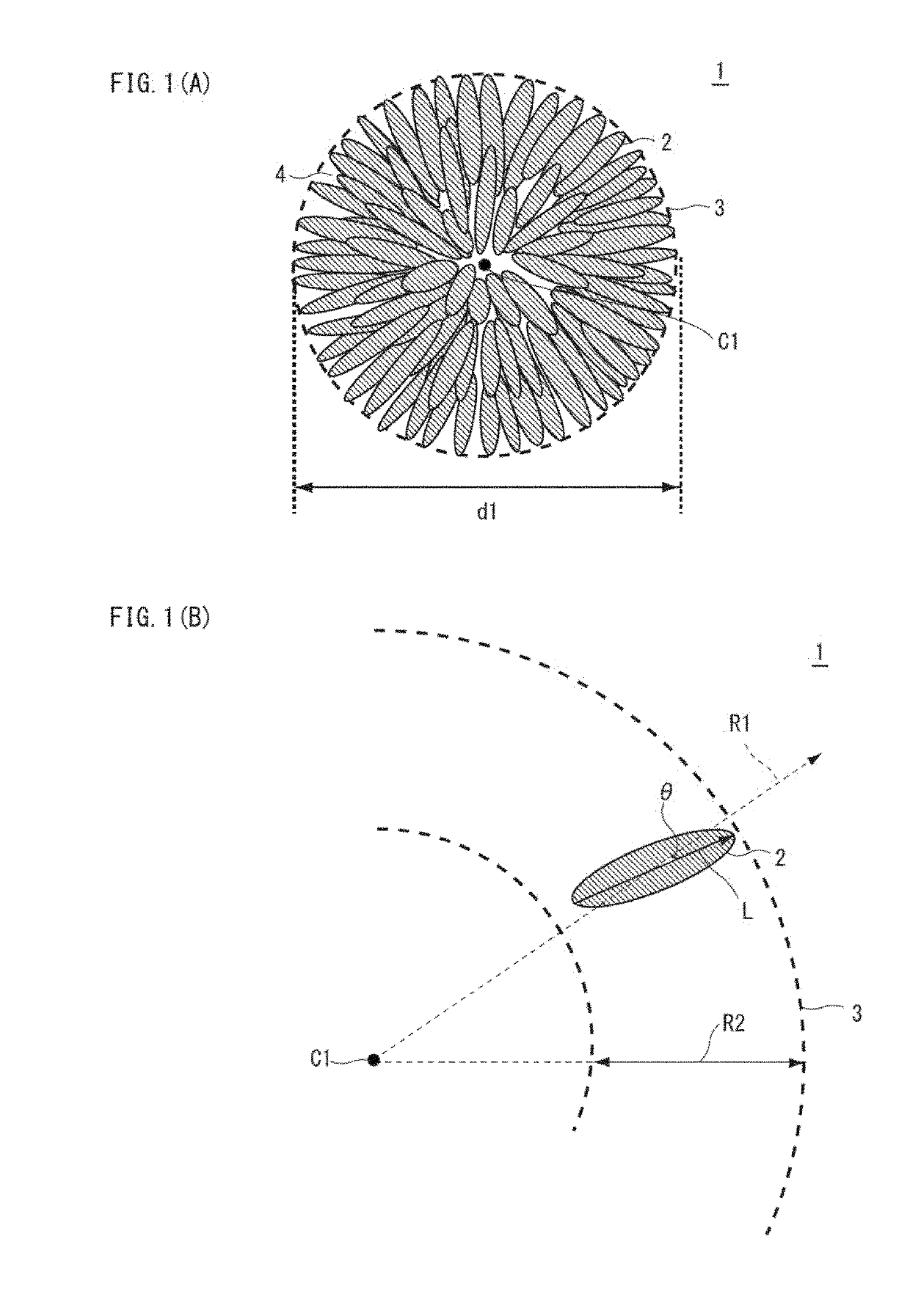
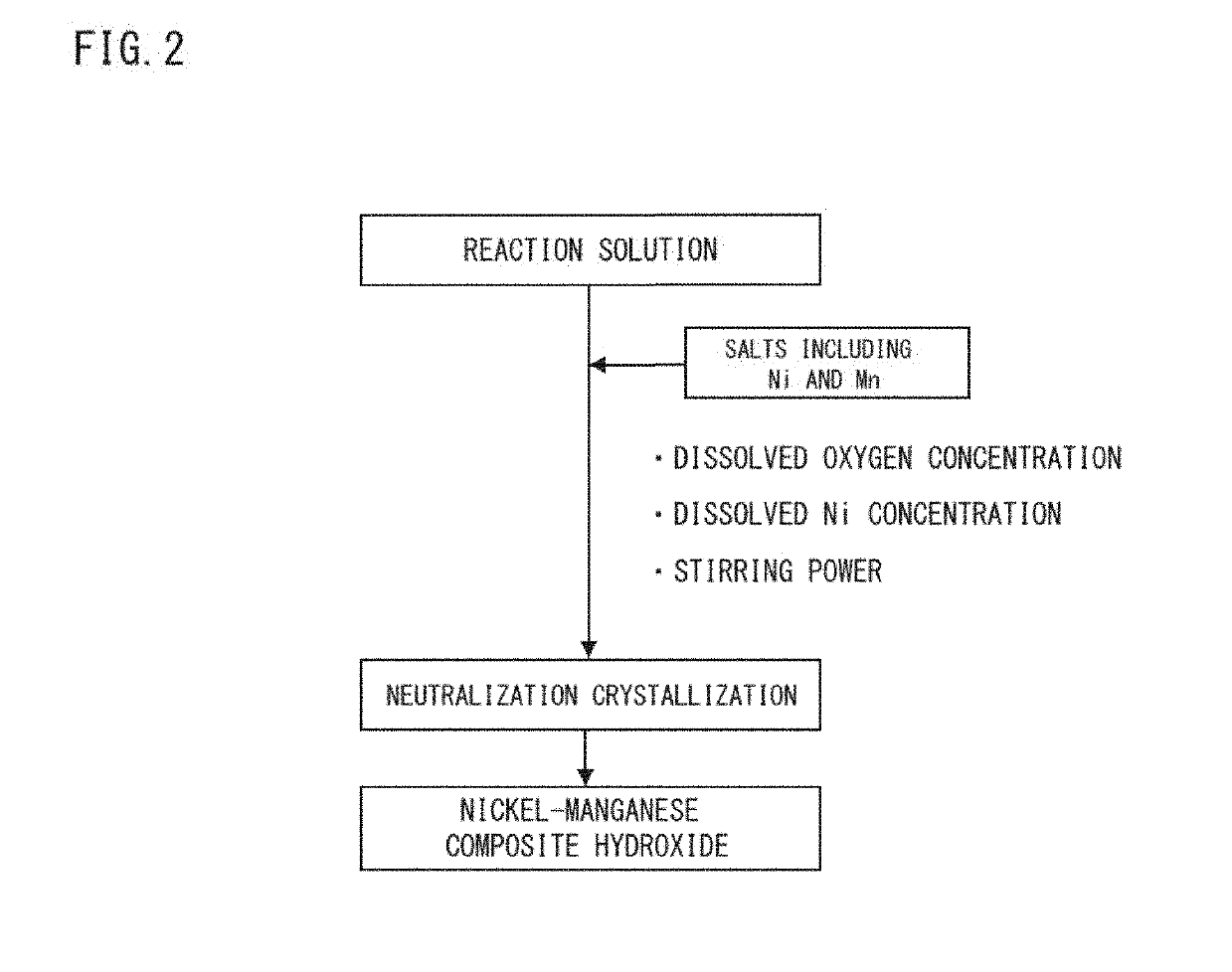
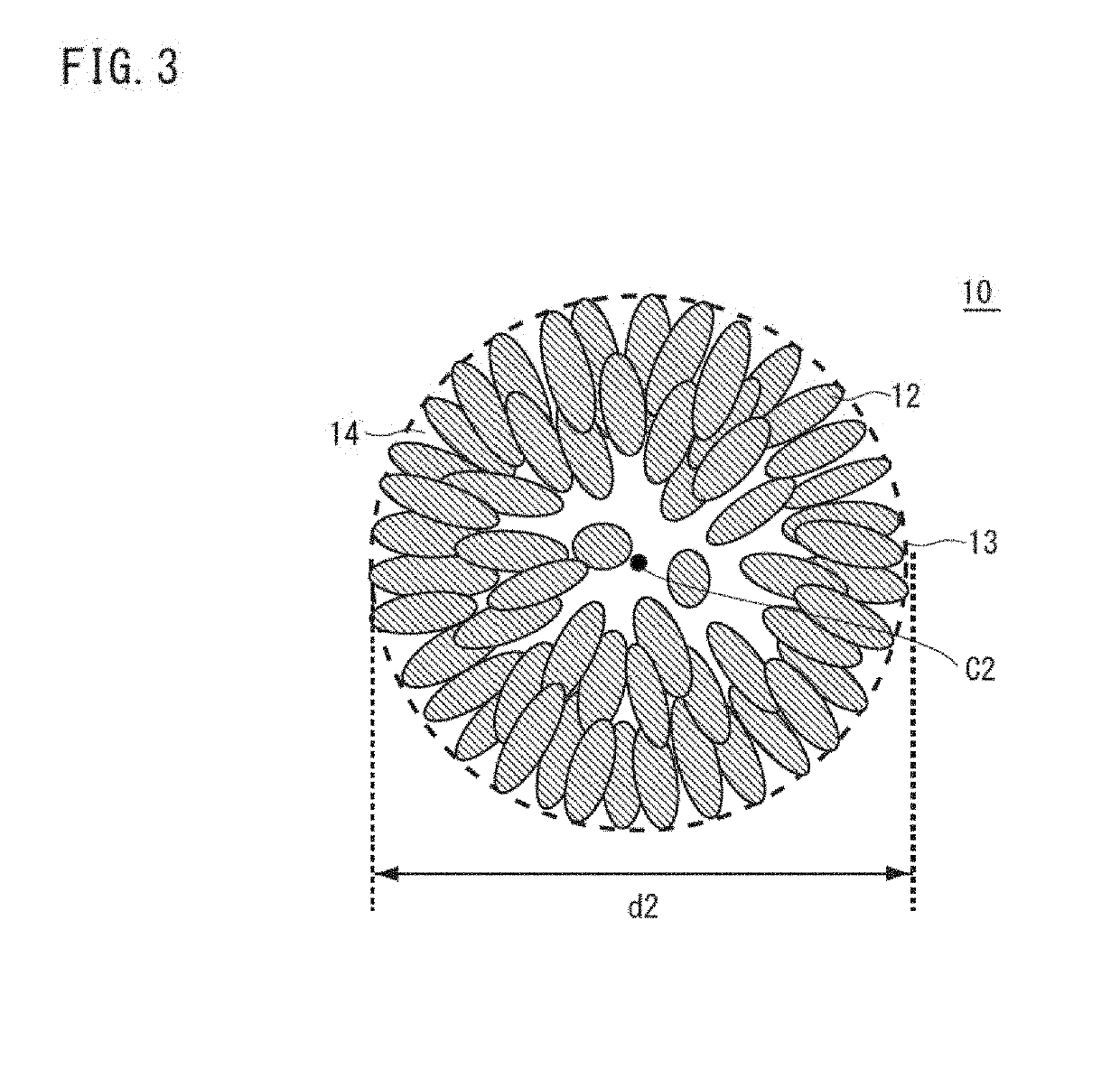
Nickel manganese composite hydroxide and method for producing same, positive electrode active material for nonaqueous electrolyte secondary battery and method for producing same, and nonaqueous electrolyte secondary battery
ActiveUS20190260024A1Increase battery capacityExcellent cycle characteristicsSecondary cellsPositive electrodesX-rayManganese
Provided are a nickel-manganese composite hydroxide capable of producing a secondary battery having a high particle fillability and excellent battery characteristics when used as a precursor of a positive electrode active material and a method for producing the same. A nickel-manganese composite hydroxide is represented by General Formula: NixMnyMz(OH)2+α and contains a secondary particle formed of a plurality of flocculated primary particles. The primary particles have an aspect ratio of at least 3, and at least some of the primary particles are disposed radially from a central part of the secondary particle toward an outer circumference thereof. The secondary particle has a ratio I(101) / I(001) of a diffraction peak intensity I(101) of a 101 plane to a peak intensity I(001) of a 001 plane, measured by an X-ray diffraction measurement, of up to 0.15.
Owner:SUMITOMO METAL MINING CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com