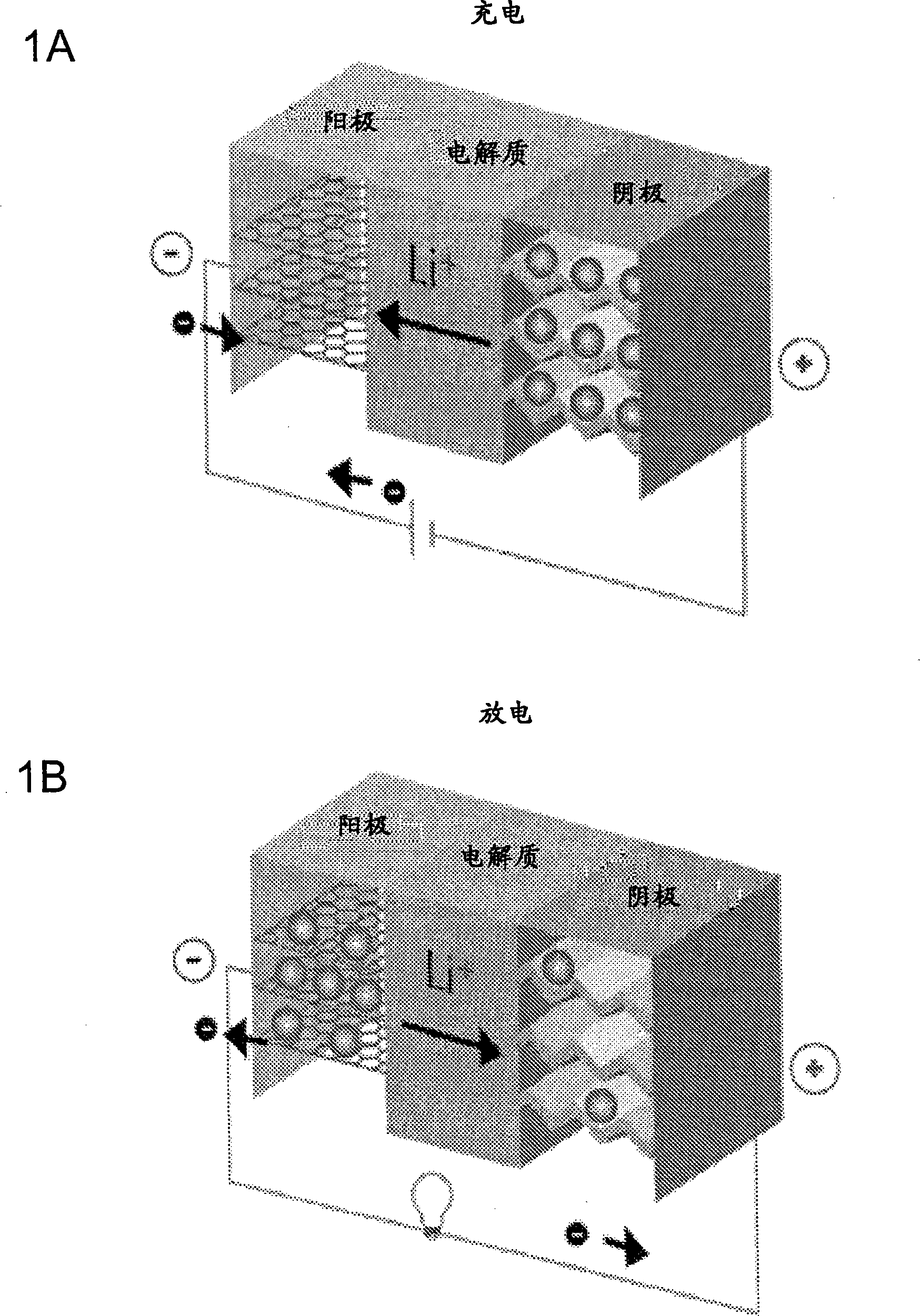
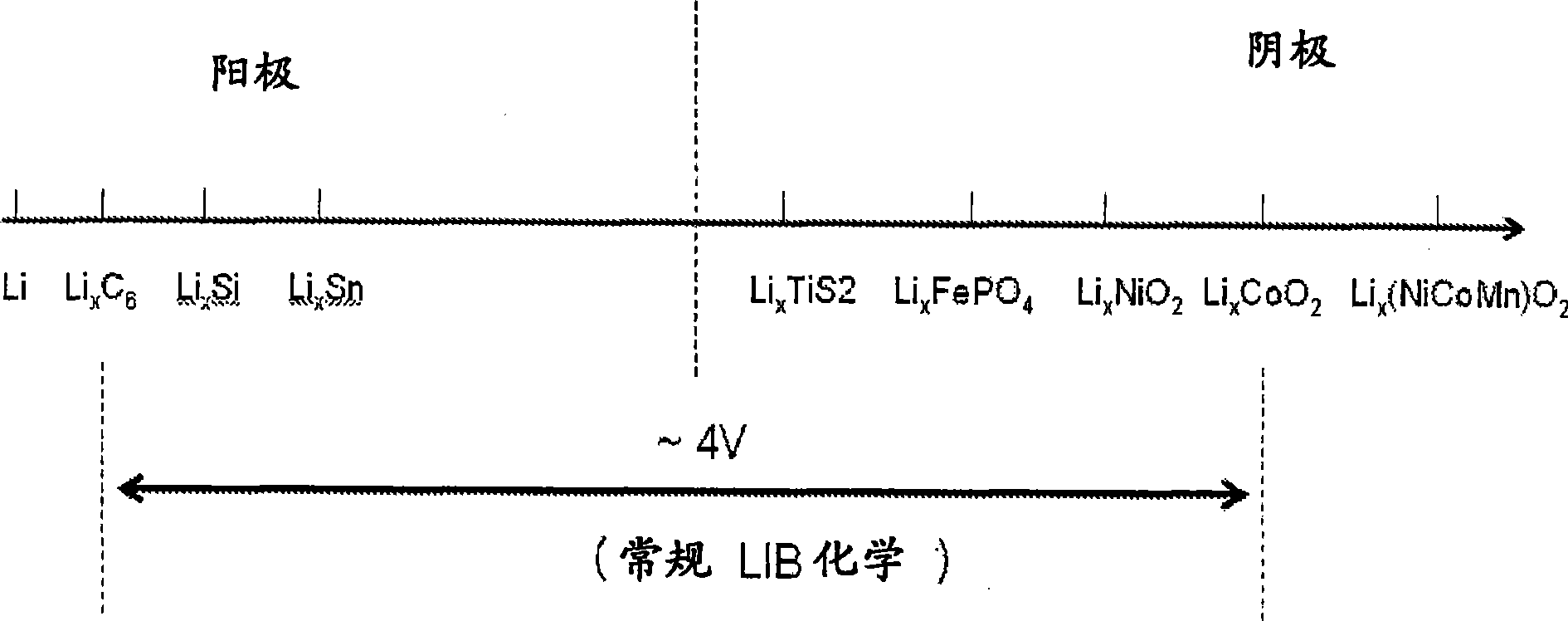
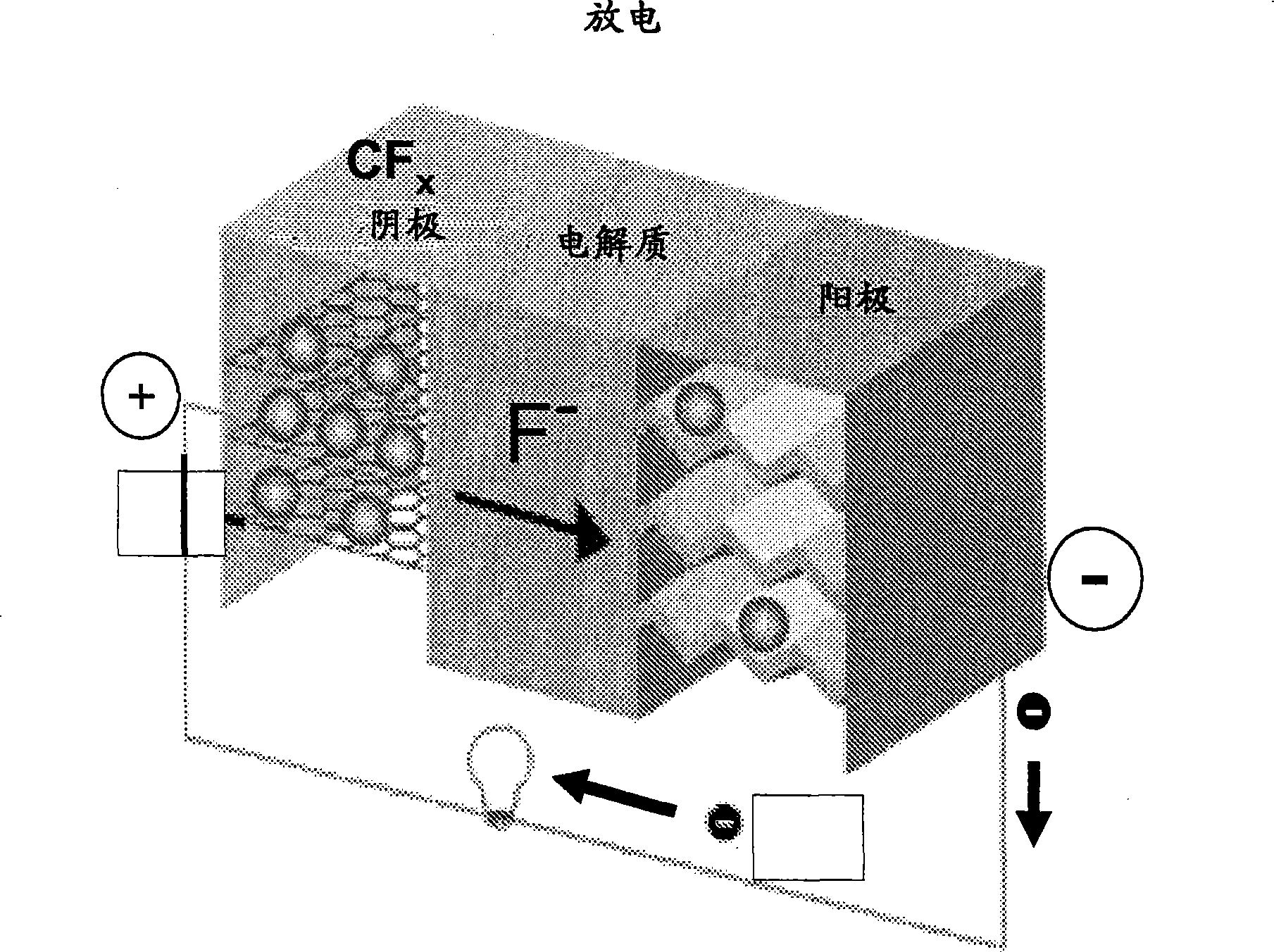
Fluoride ion electrochemical cell
An electrochemical and fluorine ion technology, applied in electrochemical generators, non-aqueous electrolyte batteries, aqueous electrolyte batteries, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Embodiment 1: Fluoride ion secondary electrochemical cell with Li / CFx half-cell structure
[0195] 1.a. Introduction
[0196] To demonstrate the advantages of the fluoride ion electrochemical cell of the present invention, a cell with a CFx positive electrode and a lithium metal negative electrode was constructed and its electrochemical performance was evaluated. The results shown here demonstrate the useful rechargeable capacity of fluoride-ion electrochemical cells at room temperature at reasonable charge-discharge rates.
[0197] 1.b. Experiment
[0198] Synthesized two classes of fluorocarbons CF x and use it as the positive electrode of the lithium battery in this example; 1) Coke-based stoichiometric (commercial) CF 1 , and 2) Subfluorinated CF based on graphite and multi-walled carbon nanotubes (MWNTs) x (x<1). Fluorocarbons are obtained by fluorinating coke graphite or MWNT carbon powder at high temperature, and the reaction is:
[0199] C(s)+x / 2F 2 (g)...
Embodiment 2
[0231] Example 2: Anion and cation acceptors for fluoride ion electrochemical cells.
[0232] This example provides a summary of anion and cation acceptors that can be used in the present invention. A variety of fluoride ion acceptors are specifically exemplified that increase the solubility of fluoride salts and that increase the ionic conductivity of the electrolyte in the electrochemical cells of the present invention.
[0233] In one embodiment, the electrolyte of the present invention includes an anion acceptor having the following chemical structure AR1:
[0234]
[0235] where R 1 , R 2 and R 3 independently selected from alkyl, aromatic groups optionally substituted with one or more halogens, alkyls, alkoxides, thiols, thioalkoxides, aromatic groups, ethers or thioethers including F , ether, thioether, heterocycle, aryl or heteroaryl.
[0236] In one embodiment, the electrolyte of the present invention includes a borate-based anion acceptor compound having th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com