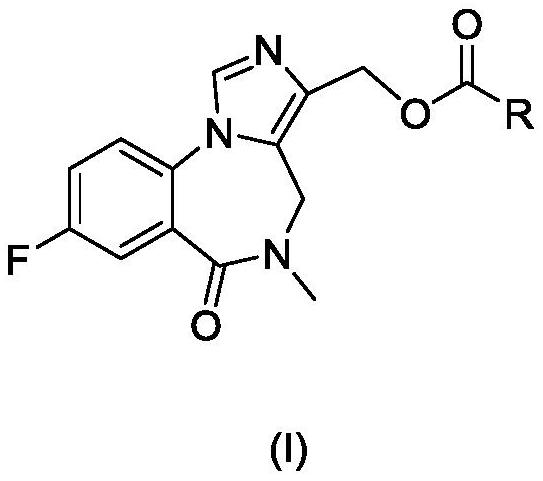
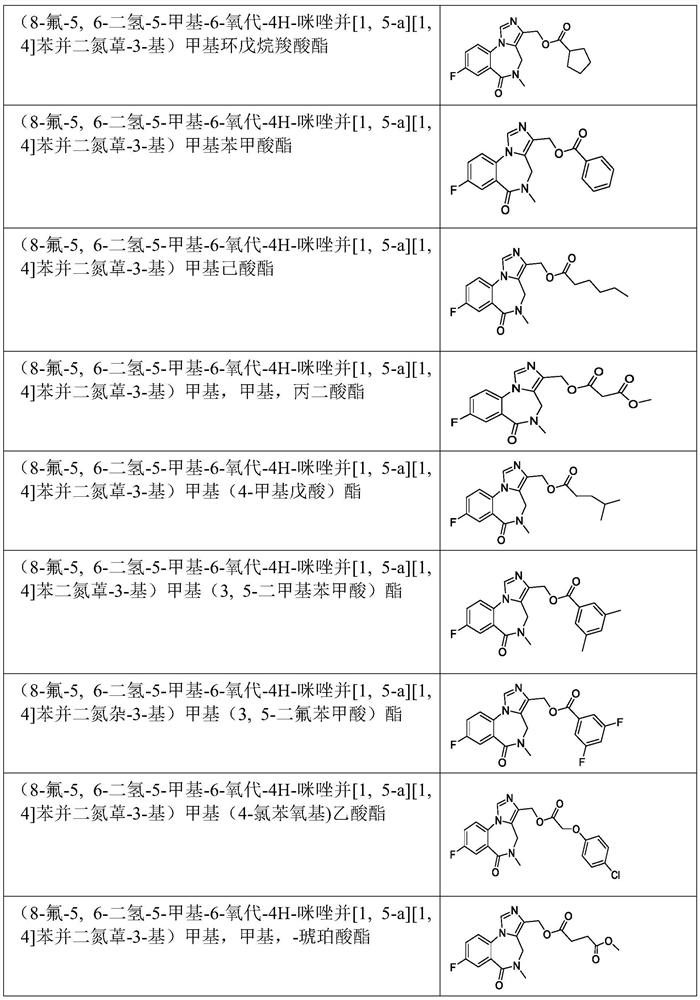
Benzodiazepine derivative and preparation method and application thereof
A benzodiazepine and derivative technology, applied in the field of pharmacy, can solve the problems of poor patient compliance, fast metabolism of flumazenil, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
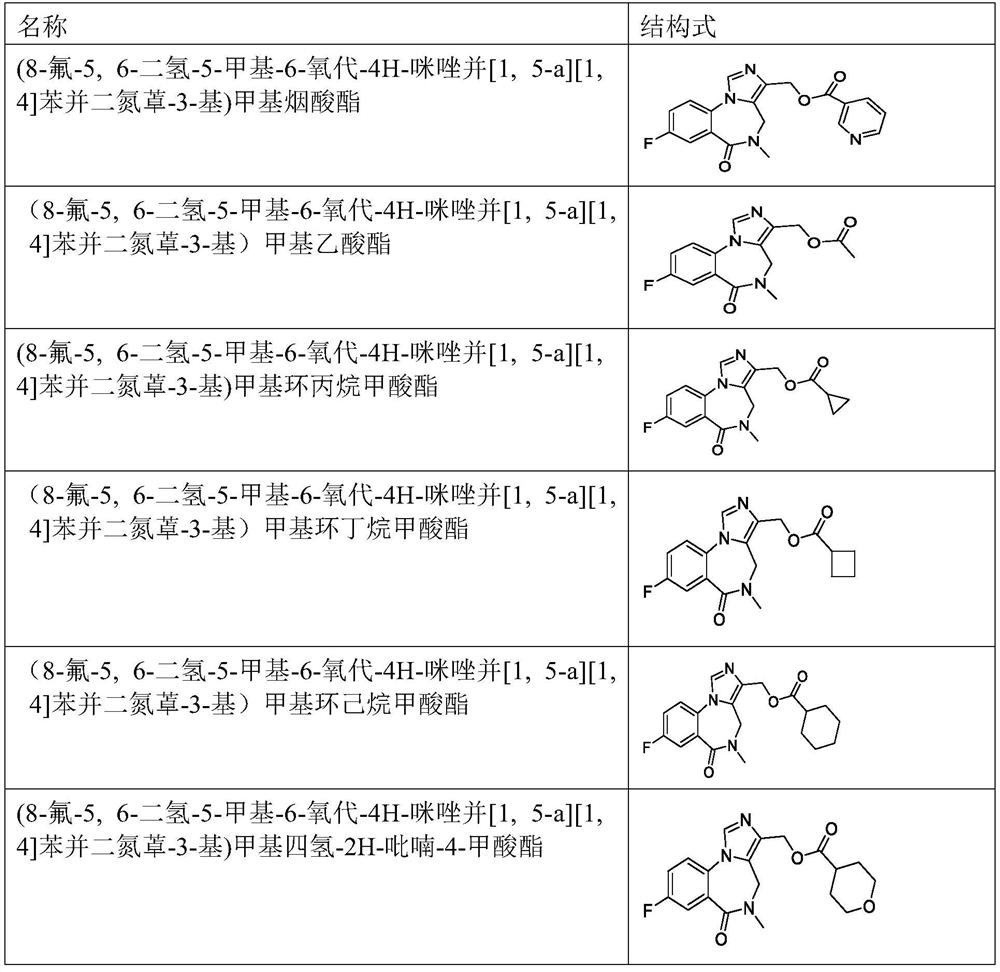
[0064] Example 1: (8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine Preparation of -3-yl) methylnicotinate (FY0348)
[0065]
[0066] (8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine The synthetic route of -3-yl) methylnicotinate is shown in the following reaction formula.
[0067]
[0068] Step a: Preparation of N-(5-fluoro-2-nitrobenzoyl)-N-methylglycine methyl ester
[0069]
[0070] Weigh 5-fluoro-2-nitrobenzoic acid (20g, 108mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI, 25g, 130mmol), 1 -Hydroxybenzotriazole (HOBT, 18g, 130mmol) was placed in a reaction flask (500ml), added dichloromethane (200ml) and stirred to dissolve, activated at room temperature for 30min; 108mmol), triethylamine (22g, 216mmol) were dissolved in dichloromethane (200ml), added to the reaction flask in sequence, and reacted at room temperature for 12h. Sampling by TLC to detect the reaction, the raw material poi...
Embodiment 2
[0085] Example 2: 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine - Preparation of 3-methyl acetate (FY0226)
[0086]
[0087] Referring to the preparation method and conditions of Example 1, 8-fluoro-3-hydroxymethyl-5,6-dihydro-5-methyl-6H-imidazo[1,5-a][1,4]benzo Dinitrogen -6-ketone and acetyl chloride were used as raw materials for esterification and condensation to obtain 136 mg of white solid, yield: 75%. 1H NMR (400MHz, chloroform-d) δ (ppm): 7.88 (s, 1H, CH), 7.73 (dd, J = 8.8, 2.9Hz, 1H, ArH), 7.38 (s, 1H, ArH), 7.35– 7.28(m,1H,ArH),5.14(s,2H,CH 2 ),4.44(s,2H,CH 2 ),3.20(s,3H.CH 3 ),2.07(s,3H,CH 3 ). HRMS (ESI, m / z) calculated value C 15 h 14 FN 3 o 3 [(M+H)+], 304.10; experimental value 304.10.
Embodiment 3
[0088] Example 3: 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3- Preparation of methyl cyclopropanecarboxylate (FY0230)
[0089]
[0090] Referring to the preparation method and conditions of Example 1, 8-fluoro-3-hydroxymethyl-5,6-dihydro-5-methyl-6H-imidazo[1,5-a][1,4]benzo Dinitrogen -6-ketone and cyclopropanoyl chloride were used as raw materials for esterification and condensation to obtain about 115 g of white solid, yield: 35%. 1H NMR (400MHz, chloroform-d) δ (ppm): 7.87 (s, 1H, CH), 7.74 (dd, J = 8.9, 2.7Hz, 1H, ArH), 7.38 (dd, J = 8.5, 4.7Hz, 1H, ArH), 7.35-7.27 (m, 1H, ArH), 5.17 (d, 2H, CH 2 ),4.43(d,2H,CH 2 ),3.20(s,3H,CH 3 ),1.65(dt,J=7.9,3.5Hz,1H,CH),1.05–0.95(m,2H,CH 2 ), 0.87 (dd, J=7.5, 3.3Hz, 2H, CH 2 ). HRMS (ESI, m / z) calculated value C 17 h 16 FN 3 o 3 [(M+H)+], 330.13; experimental value 330.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com