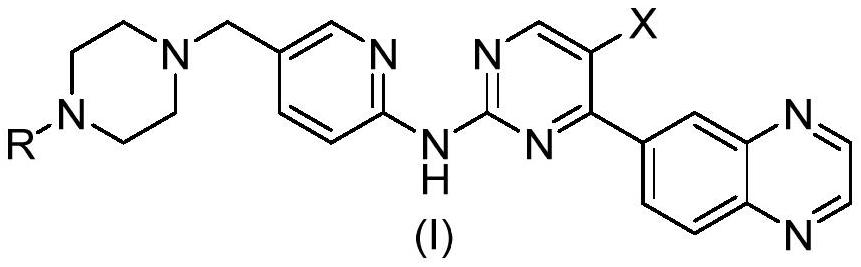
Novel CDK4/6 inhibitor as well as preparation method and application thereof
A C3-C6, C2-C6 technology, applied in the field of CDK4/6 inhibitor compounds, can solve the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1,6-(2-chloro-5-fluoropyrimidin-4-yl)quinoxaline
[0046] The first step: Synthesis of 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoxaline
[0047]
[0048] 6-bromoquinoxaline (0.42g, 2.0mmol) was dissolved in DMF (10mL), then added pinacol borate (0.53g, 2.1mmol), Pd(dppf)Cl 2 (22mg, 0.06mmol), potassium acetate (0.59g, 6.0mmol), argon was replaced three times, heated to 80°C, and reacted for 24h. Cooling, filtration, concentration column chromatography (PE / EA=10:1) gave compound 6-( 2-Chloro-5-fluoropyrimidin-4-yl)quinoxaline, white solid (0.43g, 84% yield).
[0049] 1 HNMR (300MHz, CDCl 3 )δ8.88–8.86(m,2H),8.61(d,J=1.2Hz,1H),8.16–8.07(m,2H),1.40(s,12H).
[0050] The second step: the synthesis of 6-(2-chloro-5-fluoropyrimidin-4-yl)quinoxaline
[0051]
[0052] Weigh compound 2,4-dichloro-5-fluoropyrimidine (0.23g, 1.4mmol) into a 250mL three-necked flask, then add Pd(PPh 3 ) 2 Cl 2 (21mg, 0.03mmol), sodium carbonate (...
Embodiment 2
[0054] The preparation of embodiment 2, 5-((4-ethylpiperazin-1-yl) methyl) pyridin-2-amine
[0055]
[0056] 2-Amino-5-formylpyridine (0.32g, 2.6mmol) and N-ethylpiperazine (0.45g, 3.9mmol) were dissolved in 1,2-dichloroethane (20mL), stirred at room temperature for 2h, then added Sodium triacetylborohydride (0.87g, 4.1mmol), stirred at room temperature for 8h, quenched by adding 1M NaOH (30mL), extracted with DCM (20Ml*3), dried over anhydrous sodium sulfate, concentrated and column chromatography (DCM / MeOH =10:1) The compound 5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-amine was obtained as a white solid (0.52 g, 91%).
[0057] 1 HNMR (300MHz, CDCl 3 )δ7.94(d, J=2.3Hz, 1H), 7.40(dd, J=8.3, 2.4Hz, 1H), 6.46(d, J=8.3Hz, 1H), 4.57(s, 2H), 3.36( s,2H),2.47–2.37(m,10H),1.07(t,J=7.2Hz,3H).
Embodiment 3
[0058] Embodiment 3, the preparation of tert-butyl 4-((6-aminopyridin-3-yl) methyl) piperazine-1-carboxylate
[0059]
[0060] Preparation of compound tert-butyl 4-((6-aminopyridin-3-yl)methyl)piperazine-1-carboxylate Reference compound 5-((4-ethylpiperazin-1-yl)methyl ) from the preparation of pyridin-2-amine.
[0061] 1 HNMR (300MHz, CDCl 3 ): δ7.94(d, J=2.3Hz, 1H), 7.40(dd, J=8.4, 2.3Hz, 1H), 6.48(d, J=8.4Hz, 1H), 4.54(s, 2H), 3.40 (t,J=5.1Hz,4H),3.36(s,2H),2.35(t,J=5.1Hz,4H),1.45(s,9H).
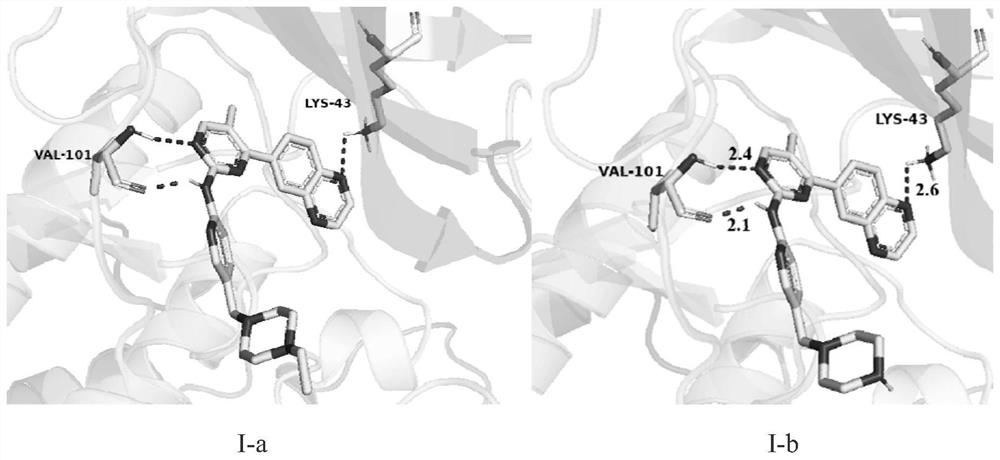
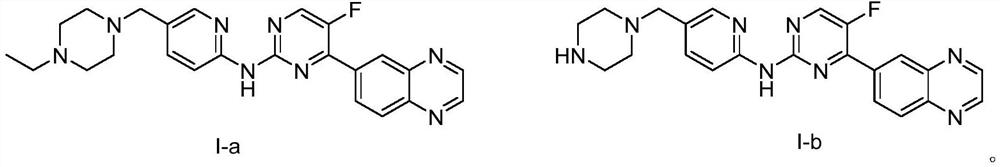
[0062] Preparation of I-a and I-b
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com