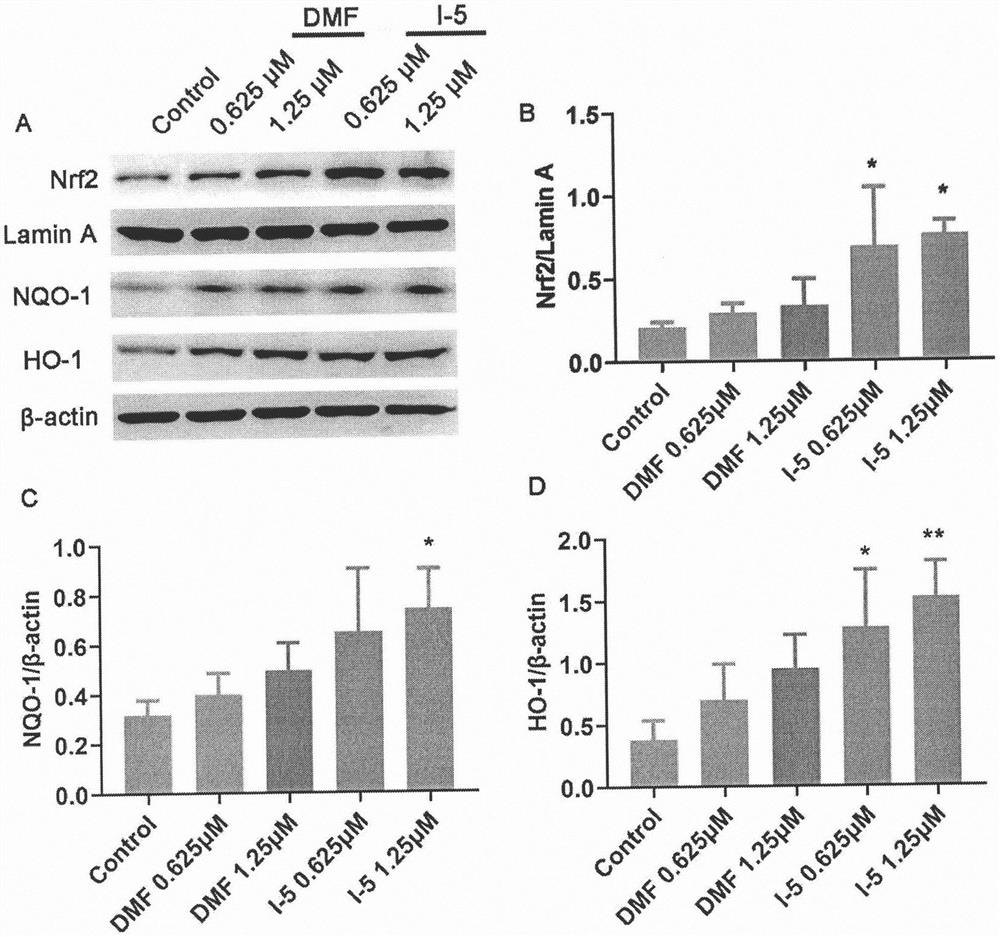
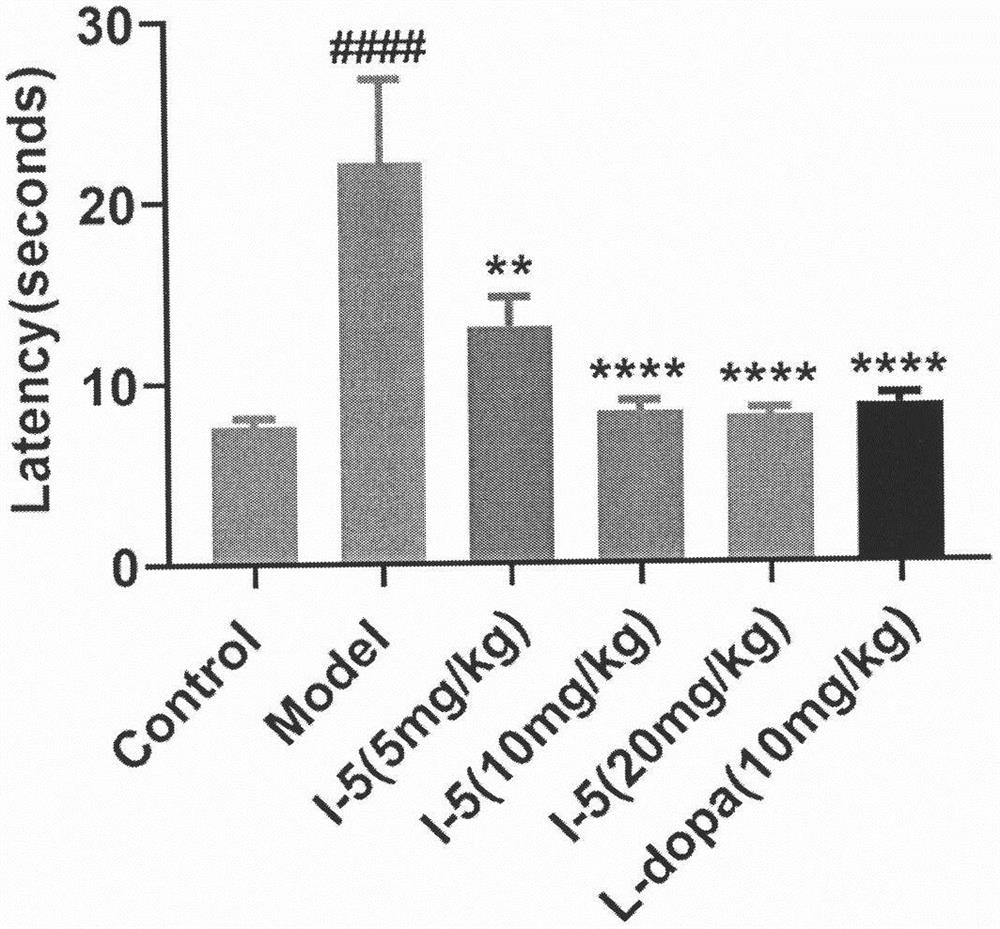
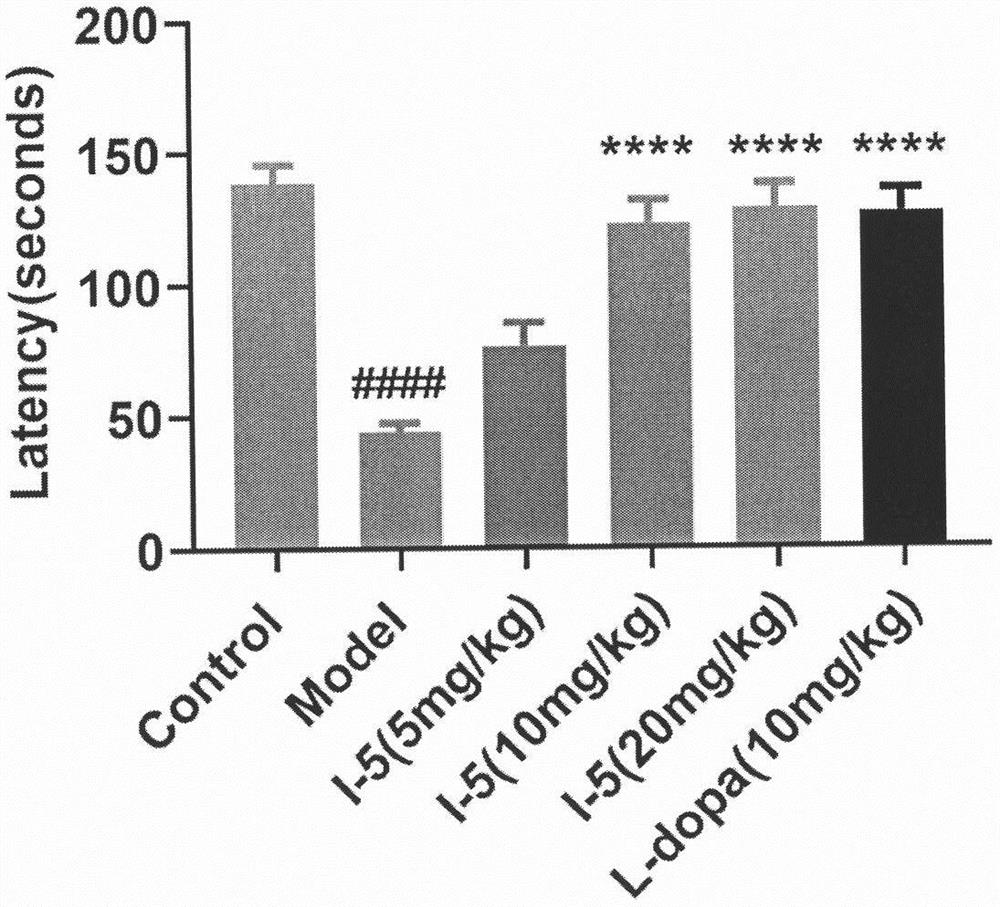
Pharmaceutical application of (E)-3-aromatic heterocyclic propyl-2-olefine acid derivative
A pharmacy, alkyl technology, applied in the field of pharmaceutical use of (E)-3-arylheterocyclylprop-2-enoic acid derivatives, can solve any problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] Steps: 0.298 g (2 mmol) pyridin-2-acrylic acid (compound a) and 10 ml of methanol in the reaction flask, slowly added 0.03 g (0.3 mmol) concentrated sulfuric acid, and the room temperature was reacted for 3 hours, and saturated saturated carbonated. The hydrogen sodium hydrogen solution regulates about 8, then extracted with dichloromethane, dried off anhydrous sulfate, filtered, concentrated, dried, 0.223 g of compound I-1, yield: 68%. 1 H NMR (CDCL 3 : Δ3.82 (S, 3H), 6.95 (D, 1H, J = 16.0 Hz), 7.28 (m, 1H), 7.43 (D, 1H, J = 8.0 Hz), 7.70 (D, 1H, J = 16.0Hz), 7.73 (m, 1H), 8.66 (D, 1H, J = 4.0 Hz); MS (ESI): M / Z 164 [M + H] + .
Embodiment 2
[0065]
[0066]Steps: 0.298 g (2 mmol) pyridine-2-acrylic acid (Compound A) and 10 ml of ethanol, slowly added 0.03 g (0.3 mmol) concentrated sulfuric acid, and saturated saturated carbonated at room temperature. The hydrogen sodium hydrogen solution is adjusted to be about 8, then extracted with dichloromethane, dried off anhydrous sulfate, filtered, concentrated, dried to give 0.249 grams of compound I-2, yield: 72%. 1 H NMR (CDCL 3 : Δ1.34 (T, 3H, J = 8.0 Hz), 4.28 (Q, 2H, J = 8.0 Hz), 6.93 (D, 1H, J = 16.0 Hz), 7.28 (M, 1H), 7.44 (D 1H, J = 8.0 Hz), 7.69 (D, 1H, J = 16.0 Hz), 7.73 (m, 1H), 8.66 (D, 1H, J = 4.0 Hz); MS (ESI): M / Z 178 [ M + h] + .
Embodiment 3
[0068]
[0069] Steps: 0.298 g (2 mmol) pyridine-3-acrylic acid (Compound A) and 10 ml of methanol in the reaction bottle, slowly drop 0.03 g (0.3 mmol) concentrated sulfuric acid, room temperature for 3 hours, drop saturated carbonated The hydrogen sodium hydrogen solution is adjusted to be about 8, then extracted with dichloromethane, dried with no water sulfate, filtrate, concentrated, dried, 0.233 g of compound I-3, yield: 71%. 1 H NMR (CDCL 3 : Δ3.75 (S, 3H), 6.44 (D, 1H, J = 16.0 Hz), 7.27 (DD, 1H, J = 4.0, 8.0 Hz), 7.61 (D, 1H, J = 16.0 Hz), 7.77 (D, 1H, J = 8.0 Hz), 8.54 (D, 1H, J = 4.0 Hz), 8.67 (S, 1H); MS (ESI): M / Z 164 [M + H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com