Pyran compound and synthesis method thereof
A synthesis method and compound technology, applied in the field of pyran compounds and their synthesis, can solve problems such as hindering the application of schemes, long reaction time, metal catalysts and high reaction temperature, and achieve excellent inhibitory activity, high application value and product yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
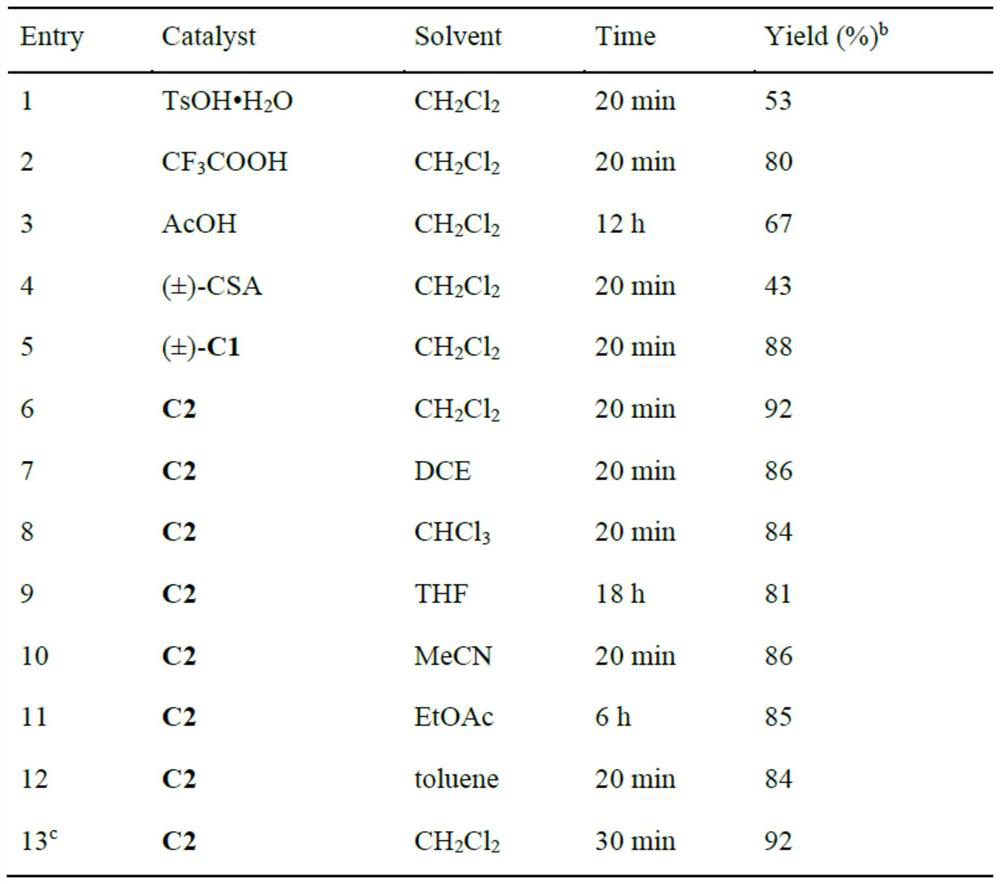
[0053] The technical solutions of the present invention will be further described below in conjunction with the embodiments. Obviously, the described embodiments are only some of the embodiments of the present invention, not all of them. Based on the embodiments of the present invention, all other embodiments obtained by those skilled in the art without making creative efforts belong to the protection scope of the present invention.
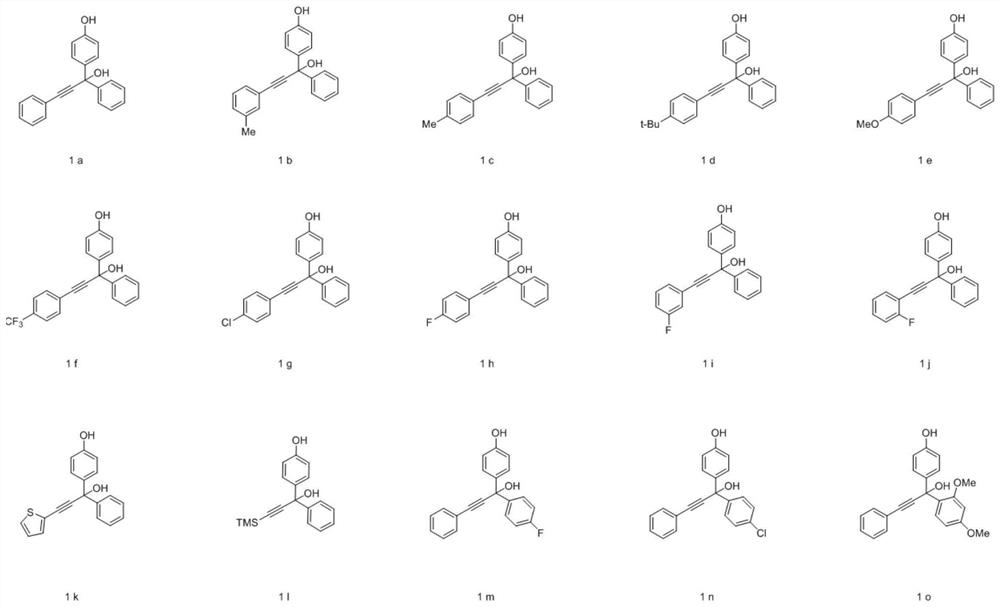
[0054] The invention provides a synthetic method of pyran compounds, specifically: through propargyl p-benzoquinone methides ((aza)-p-QMs), and 4-hydroxycoumarin and its derivatives or 1 , 3-diketone compounds are prepared by (3+3) cycloaddition reaction, the specific reaction formula is as follows:
[0055]
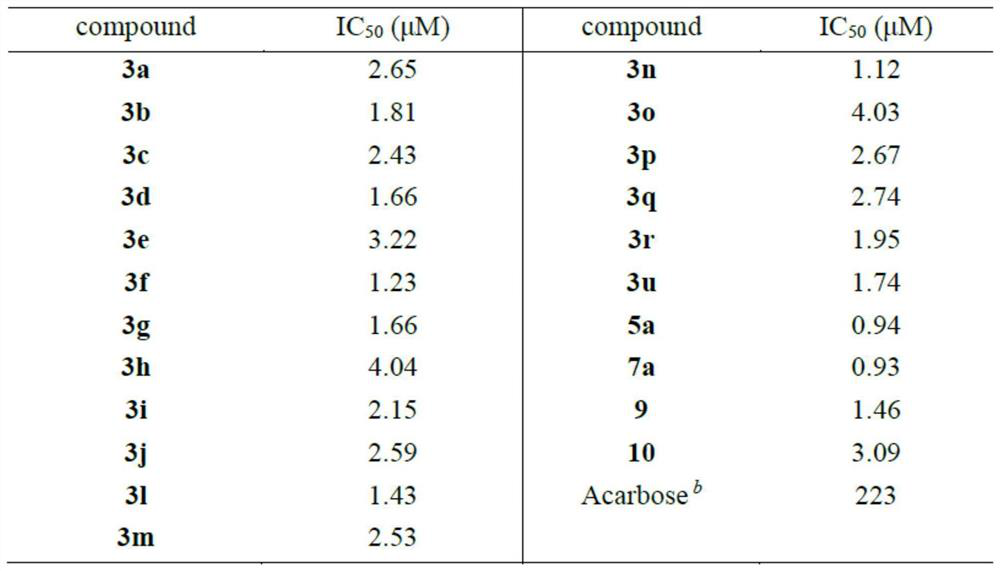
[0056] The method provided by the present invention can efficiently and rapidly synthesize a series of pyran compounds with rich functional groups and novel structures under relatively mild conditions. According to the research on the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com