Multi-metal non-oxide electrocatalyst as well as preparation method and application thereof
A technology for oxide electrolysis and catalyst, which is applied to electrodes, electrolysis process, electrolysis components, etc., can solve the problems of easy aggregation of catalysts, limited effect, limited effective electroactive sites, etc., and achieves simple and controllable preparation method and improved reaction rate. , to achieve the effect of application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
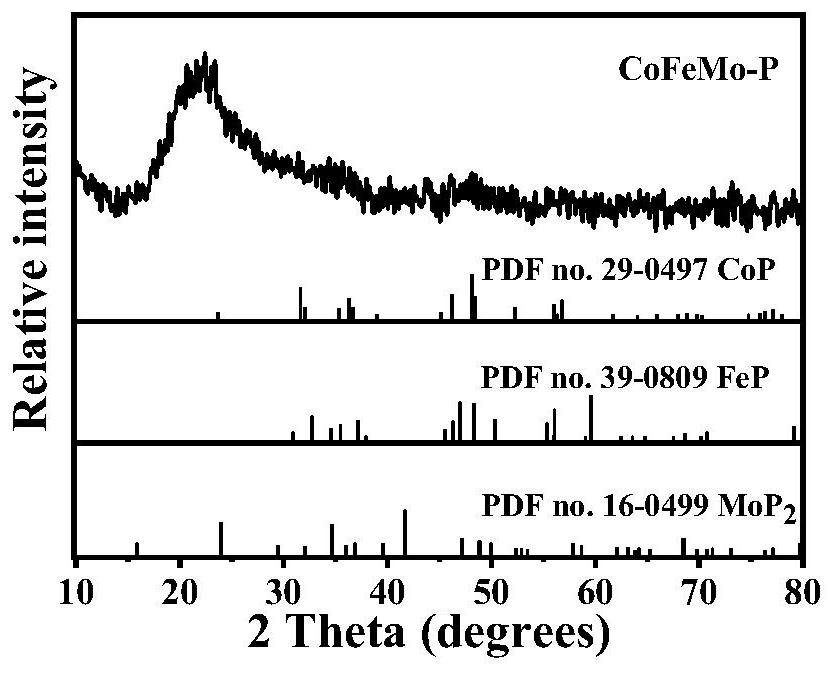
[0031] Step 1, the preparation of precursor ZIF-67: 0.87gCo(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of methanol, and the solution was injected into 20 mL of methanol containing 1.97 g of 2-methylimidazole under stirring, and the purple precipitate obtained after standing at room temperature for 24 h was centrifuged and washed 3 times with absolute ethanol, Then it was dried in vacuum at 70°C for 10 h to obtain ZIF-67.
[0032] Step 2, Synthesis of Co-Fe layered double hydroxide (CoFe-LDH):
[0033] First disperse 0.08g of ZIF-67 in 40mL ethanol to obtain ZIF-67 alcohol dispersion;
[0034] then in N 2 Under the atmosphere, mix 20mL ethanol with 0.18gFeCl 2 4H 2 5 mL of deionized aqueous solution of O was quickly poured into the above-mentioned ZIF-67 alcohol dispersion at room temperature, stirred for 30 min, centrifuged to obtain a precipitate, washed with ethanol three times, and vacuum-dried at 70 °C for 10 h to obtain CoFe-LDH.
[0035] Step 3, Synthesis of Co-Fe-Mo...
Embodiment 2
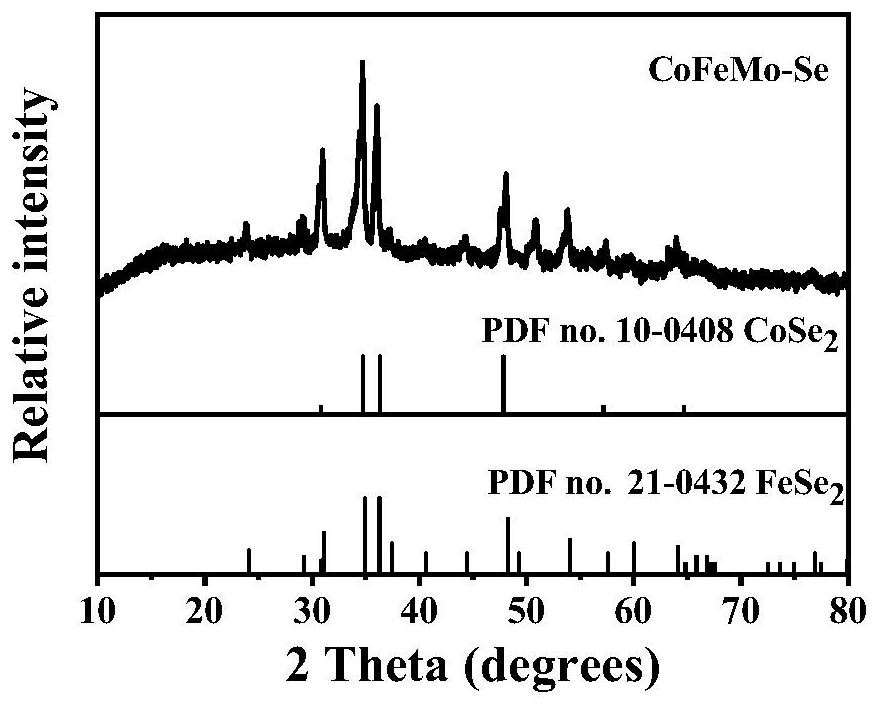
[0043] Step 1, the preparation of precursor ZIF-67: 0.87gCo(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of methanol, and the solution was injected into 20 mL of methanol containing 1.97 g of 2-methylimidazole under stirring, and the purple precipitate obtained after standing at room temperature for 24 h was centrifuged and washed 3 times with absolute ethanol, Then it was dried in vacuum at 70°C for 10 h to obtain ZIF-67.
[0044] Step 2, Synthesis of Co-Fe layered double hydroxide (CoFe-LDH):
[0045] First, disperse 0.08 g of ZIF-67 in 36 mL of ethanol to obtain a ZIF-67 alcohol dispersion;
[0046] then in N 2 Under the atmosphere, mix 20mL ethanol with 0.18gFeCl 2 4H 2 4.5 mL deionized aqueous solution of O was quickly poured into the above ZIF-67 alcohol dispersion at room temperature, stirred for 30 min, centrifuged to obtain a precipitate, washed with ethanol three times, and vacuum dried at 65 °C for 12 h to obtain CoFe-LDH.
[0047] Step 3, Synthesis of Co-Fe-Mo la...
Embodiment 3
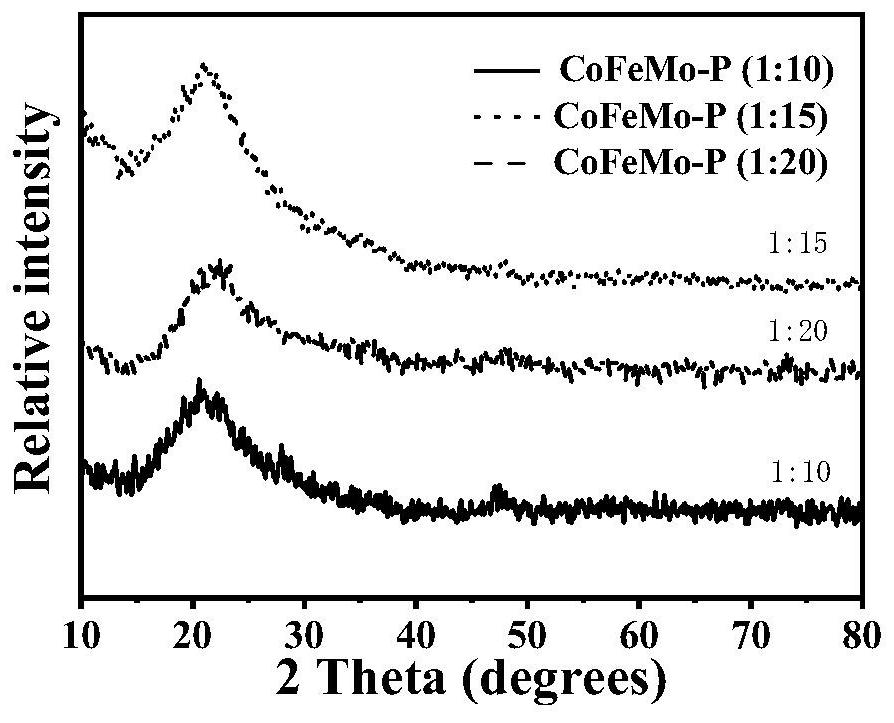
[0055] Step 1, the preparation of precursor ZIF-67: 0.85gCo(NO 3 ) 2 ·6H 2 O was dissolved in 28 mL of methanol, and the solution was poured into 18 mL of methanol containing 1.95 g of 2-methylimidazole under stirring, and the purple precipitate obtained after standing at room temperature for 18 h was centrifuged and washed twice with absolute ethanol. It was then dried in vacuum at 68°C for 11 h to obtain ZIF-67.
[0056] Step 2, Synthesis of Co-Fe layered double hydroxide (CoFe-LDH):
[0057] First, disperse 0.08 g of ZIF-67 in 42 mL of ethanol to obtain a ZIF-67 alcohol dispersion;
[0058] then in N 2 Under the atmosphere, mix 20mL ethanol with 0.18gFeSO 4 ·6H 2 5 mL of deionized aqueous solution of O was quickly poured into the above ZIF-67 alcohol dispersion at room temperature, stirred for 30 min, centrifuged to obtain a precipitate, washed with ethanol three times, and vacuum-dried at 70 °C for 12 h to obtain CoFe-LDH.
[0059] Step 3, Synthesis of Co-Fe-Mo laye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap