Compositions and methods for purification of metals from steel making waste streams
A metal and mixture technology, applied in recycling technology, process efficiency improvement, calcium/strontium/barium chloride, etc., can solve problems such as volatile ammonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
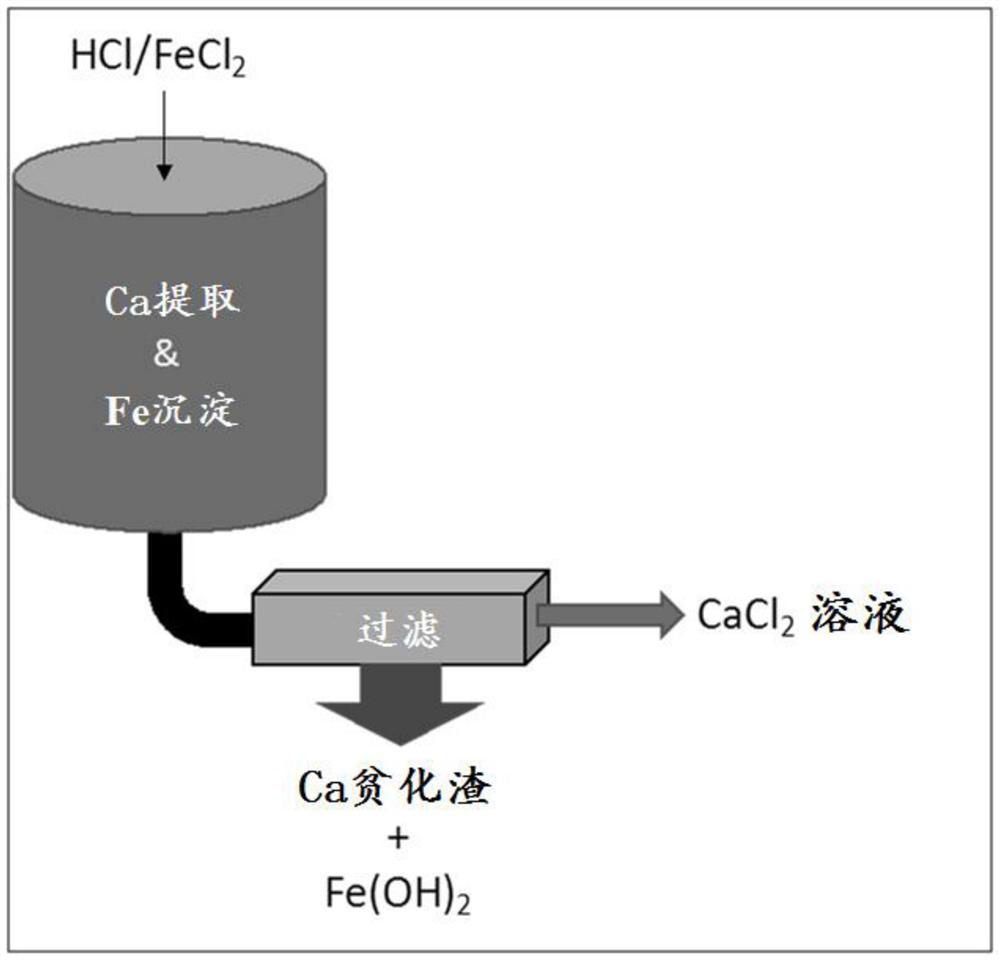
[0076] Example 1: A simulated spent acid wash solution was prepared by adding 54.1 g of 37% HCl to 83.2 g of water. Add 62.7g FeCl to the HCl solution 2 2H 2 O, stir the mixture until the ferrous chloride is completely dissolved. The resulting solution was medium green (much like pickle juice) and contained 10 wt% HCl and 20 wt% FeCl 2 .
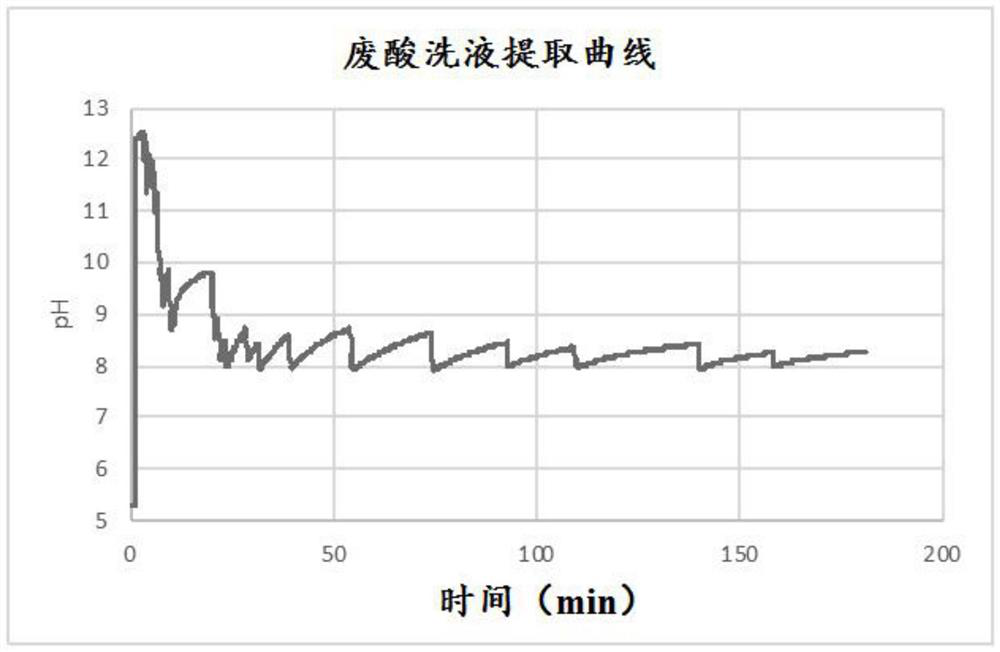
[0077] Into a 500 mL beaker was added 300 g of water and 2.00 g of monoethanolamine hydrochloride (MEACl) chosen as lixiviant. The resulting solution was magnetically stirred at 500 rpm and a pH probe with a data logger was placed in the solution. The pH of the solution was about 5.3. 30 g of BOF slag ( image 3 shown. As the reaction progressed, the color of the slurry was observed to change from dark brown (typical slag color) to dark green, indicating the formation of Fe 2 (OH) 2 solid. The total amount of the added simulated waste pickling solution is 55.96g.
[0078] After extracting the required amount of calcium, the mixture ...
Embodiment 2
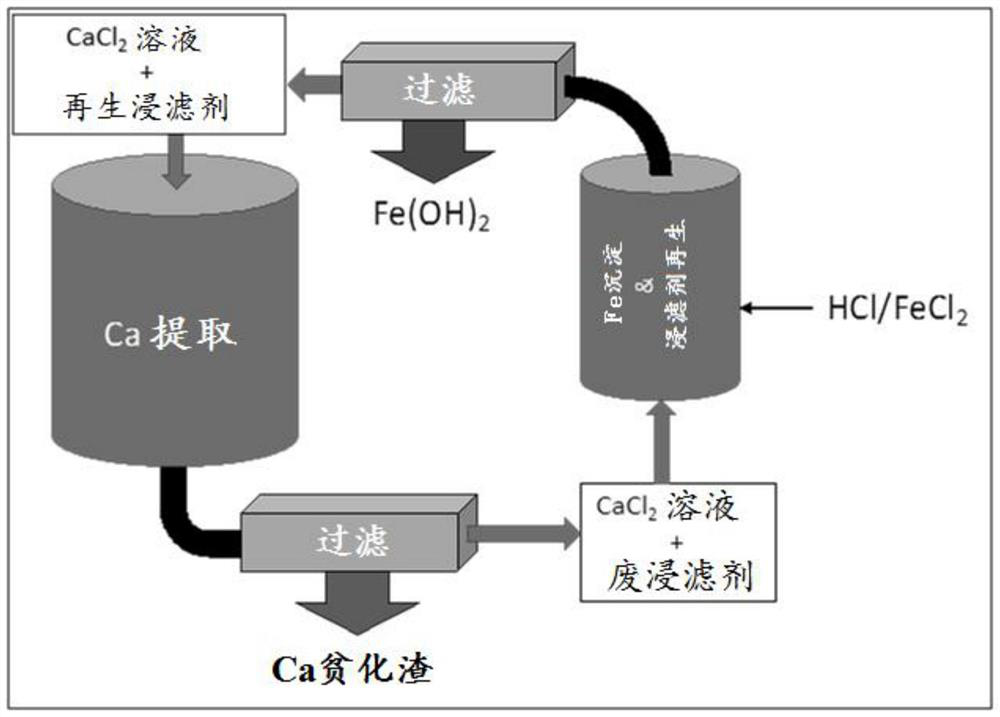
[0080] Example 2: In a 500mL beaker, 1.25g of MEA was diluted into 300g of water, and the solution was magnetically stirred at a speed of 500rpm. To the stirred solution was added 1 chloride equivalent (4.75 g) relative to MEA of the simulated spent acid wash (prepared in Example 1). A dark green precipitate formed, consistent with the formation of ferrous hydroxide. The pH of the measured solution was about 8. With constant agitation, an additional 2.73 g of simulated spent acid wash was added to bring the total acid to MEA parity. The solution re-cleared yellow / orange and had a pH of 3-4.
Embodiment 3
[0081] Example 3: In a 500mL beaker, 1.25g of MEA was diluted into 300g of water, and the solution was magnetically stirred at a speed of 500rpm. Add 6.0g CaCl to it 2 2H 2 O to provide partially extracted CaCl similar to that described in Example 1 2 Loading (1.5%). To the stirred solution was added 1 chloride equivalent (4.75 g) relative to MEA of the simulated spent acid wash (prepared in Example 1). A dark green precipitate formed, consistent with the formation of ferrous hydroxide. With constant agitation, an additional 2.73 g of simulated spent acid wash was added to bring the total acid to MEA parity. The solution re-clarified and the resulting solution was yellow / orange.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com