Engineered microorganisms with g3p---> 3pg enzyme and/or fructose-1,6-bisphosphatase including those having synthetic or enhanced methylotrophy
A technology of methyl nutrition and microorganisms, applied in the fields of biochemical equipment and methods, biofuels, enzymes, etc., can solve the problems of unsuitability for genetic engineering, unfavorable industrial production, and few metabolic intermediates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
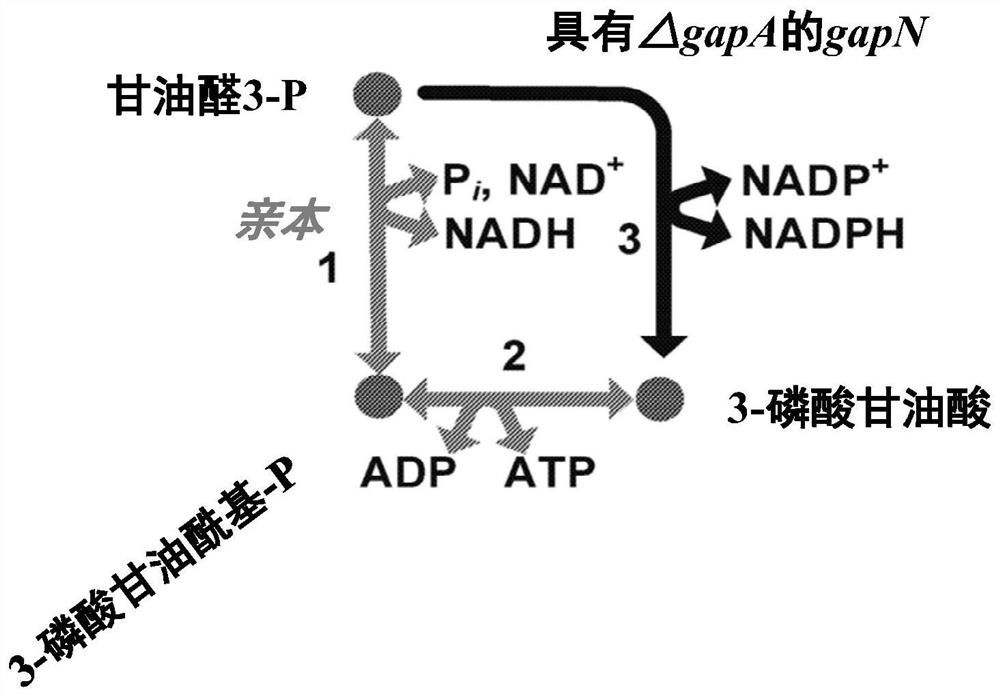
[0303] Example 1-GAPN and GAPA dynamics contrast
[0304] The catalytic constant of E. coli GAPA and the GAPN of Bacillus Methanol was measured and shown in Table 18.
[0305] Table 18.
[0306]
Embodiment 2
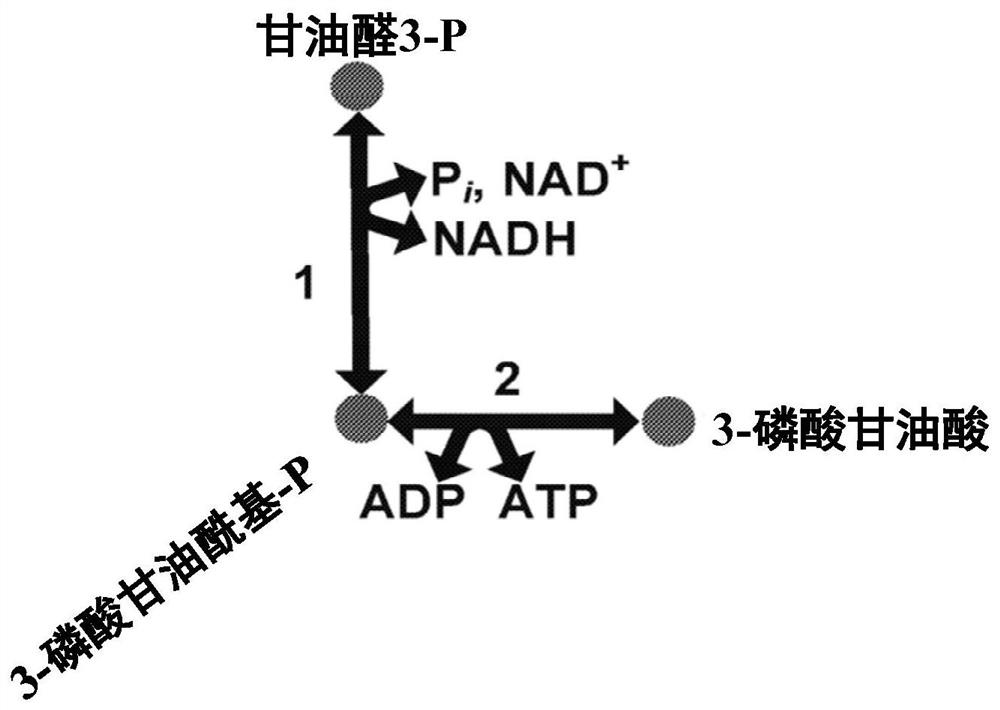
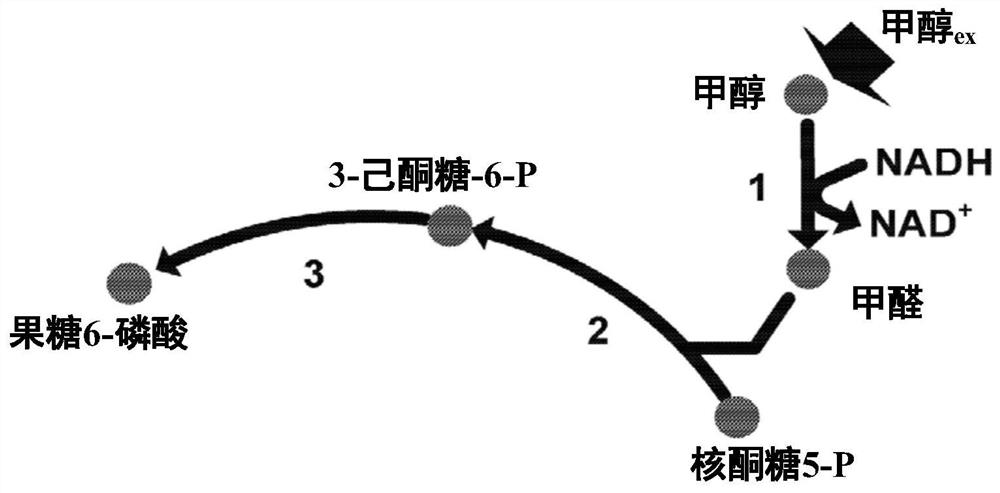
[0307] Example 2 - Due to GAPN expression to obtain intracellular metabolism
[0308] The intracellular metabolic production spectrum of Escherichia coli, of which GAPN enzyme (SEQ ID: 1 having 95% ID) and hex ketosyl-6-phosphatase (HPS) with SEQ ID: 1-phosphatase (HPS) (SEQ ID: 2) Combination, 6-phosphate-3-hexazone isomerase (PHI) (SEQ ID: 3) was compared to strains expressing Gapa and expressed the same HPS and PHI enzymes. The strain also lacks GAPA, expresses methanol dehydrogenase, and expresses FBA, GLPX, RPE, and TKTT from Bacillurbium. The expression of GAPN results in an increase in the metabolites of the related RUMP circulating metabolites compared to strains with GAPA expression without GAPN. This suggests that GAPN expression is the benefit of RUMP cycle activity and methyl nutrition. Table 19 shows the multiple variations of the key RUMP circulating metabolies needed to be related to methyl nutrition.
[0309] Table 19: The multiplication of RUMP circulating metabol...
Embodiment 3
[0311] Example 3 - Synthetic Methyl Nutrition in Culture
[0312] Escherichia coli strain (overexpression of GAPN (login number WP_003351798; with SEQ ID: 1 with 95% ID), Gapa deletion, other enzymes, methanol dehydrogenase, HPS (SEQ ID: 2), PHI (SEQ ID: 3 ), FBA, GLPX, TKT) and its parent strains expressing GAPA culture were cultured in a constant medium containing glucose and methanol. The constant bioreactor is initiated in a batch culture mode and operated under aerobic conditions, with glucose is carbon source, temperature control at 35 degrees Celsius, and the oxygen is controlled in 20% or more, and the gas flow is changed and stirred. The pH is constant at 6.95 by automatically adding base or acid when necessary. By a dilution rate of 0.1H-1, a medium containing methanol as a single carbon source is performed, and the fermenter is continuously operated, and the transition to methanol is performed. The methanol feed was started before glucose depletion, and the methanol gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com