Anti-skin tumor compound and application thereof
A technology of skin tumors and compounds, applied in the field of anti-tumor drugs, to achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method comprises the following steps: react compound 1, compound 4, azidotrimethylsilane (TMSN3) and iodobenzene bistrifluoroacetate in an organic solvent to obtain the anti-skin tumor compound A. Preferably, the above-mentioned organic solvent is benzene, and the reaction preparation yield in benzene is higher.
[0045] When R in the above anti-skin tumor compound 2 When selected from -CHOH-, the reaction principle is as follows:
[0046]
[0047] The preparation method comprises the following steps: reducing the anti-tumor compound A with a reducing agent to obtain the anti-skin tumor compound B. Optionally, the reducing agent is sodium borohydride and / or lithium aluminum tetrahydride.
[0048] When R in the above anti-skin tumor compound 2 selected from -C(CH 2 )-, the reaction principle is as follows:
[0049]
[0050] The preparation method comprises the following steps: mixing and stirring methyltriphenylphosphine bromide, THF and NaOtBu...
Embodiment 1
[0063]
[0064] Compound 1 (888mg, 5mmol), compound 2 (1.16g, 20mmol) and TMSN 3 (1.15g, 10mmol) was dissolved in 30mL of benzene, and iodobenzene bistrifluoroacetate (4.3g, 10mmol) was added in portions within 5-10 minutes at room temperature. After stirring at room temperature for 24 hours, Et 3 N (12.5 mL), stirred for 10 minutes. The solvent was removed under reduced pressure and purified by column chromatography to obtain compound 3 (806 mg, 69%). 1 H NMR (500MHz, CDCl 3 )δ8.23(d, J=8.9Hz, 1H), 8.07(s, 1H), 7.52(d, J=9.0Hz, 1H), 7.46(s, 1H), 3.06(q, J=7.0Hz, 2H), 2.80(s, 3H), 1.30(t, J=7.1Hz, 3H); HRMS found: 234.0687.
[0065] Compound 3 (58 mg, 0.25 mmol) was dissolved in 3.1 mL of EtOH, and NaOH (50 mg, 1.25 mmol) was added. After stirring for 5 minutes, compound 4 (63 mg, 0.3 mmol) was added. After the reaction was complete, the mixture was extracted with EtOAc, then the combined organic layers were washed with saturated brine, washed with anhydrous Na 2 SO ...
Embodiment 2
[0068]
[0069] Dissolve 2-methyl-7-chloroquinoline (139mg, 0.785mmol), 3-formaldehyde-9-methylcarbazole (657mg, 3.14mmol), azidotrimethylsilane (181mg, 1.57mmol) in 1.5mL Add iodine bistrifluoroacetate (675mg, 1.57mmol) in batches to benzene within 10 minutes, stir at room temperature for 24 hours, add 0.5mL triethylamine and stir for 10 minutes, concentrate, and obtain A (170mg, 56% ). The NMR spectrum is: 1 H NMR (500MHz, CDCl 3 )δ8.56(s,1H),8.13(s,1H),8.05(d,J=7.8Hz,1H),8.00(d,J=8.6Hz,1H),7.75(d,J=8.9Hz, 1H), 7.55(t, J=7.6Hz, 1H), 7.48-7.41(m, 2H), 7.40-7.36(m, 2H), 7.30(t, J=7.6Hz, 1H), 3.91(s, 3H ),2.81(s,3H); 13 CNMR (125MHz, CDCl 3 )δ195.1, 159.7, 148.7, 146.1, 144.4, 141.8, 135.8, 128.3, 128.2, 127.9, 127.4, 127.0, 126.8, 124.0, 122.91, 122.87, 122.1, 120.8, 120.2, 19.5, 19.0 The resolution mass spectrum HRMS is: 385.1099, 387.1075.
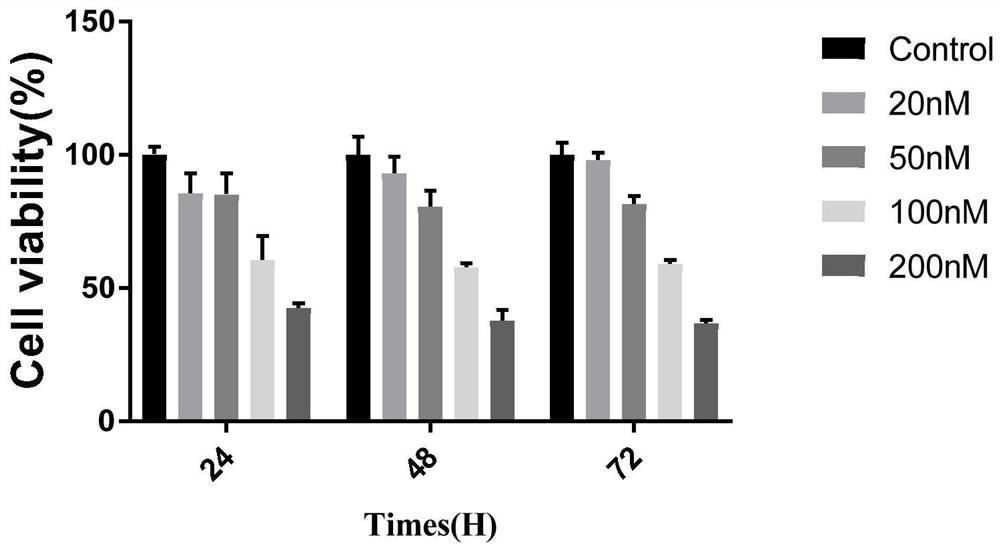
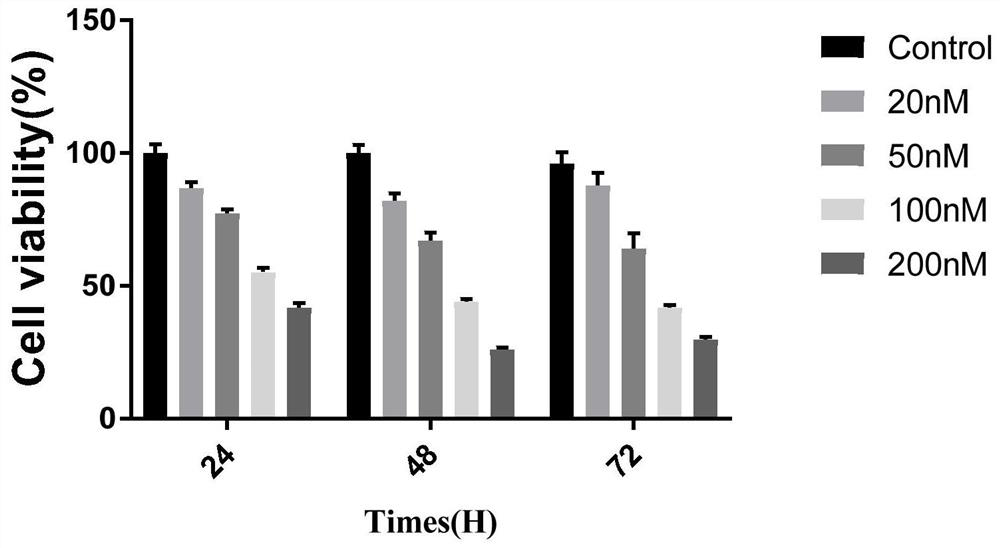
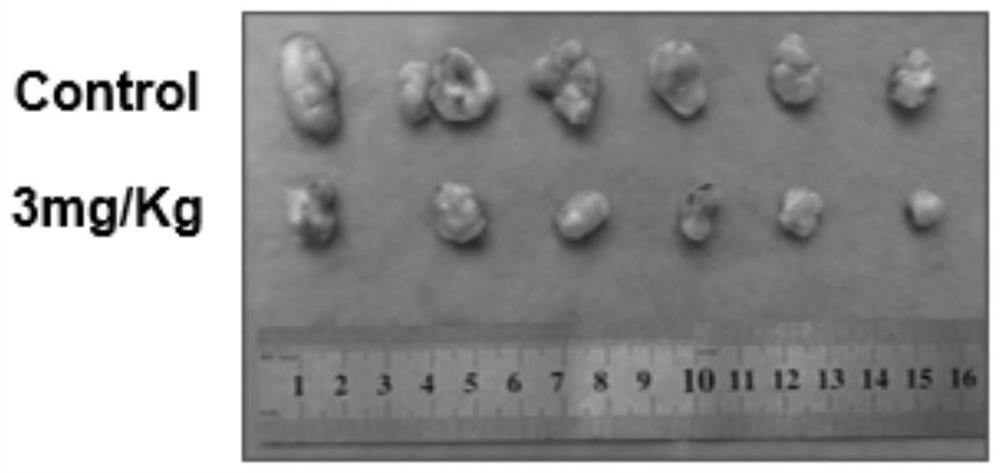
[0070] Use the compound obtained in Example 2 to carry out CCK-8 test: get human skin malignant melanoma cell SK-Mel-5, make ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com