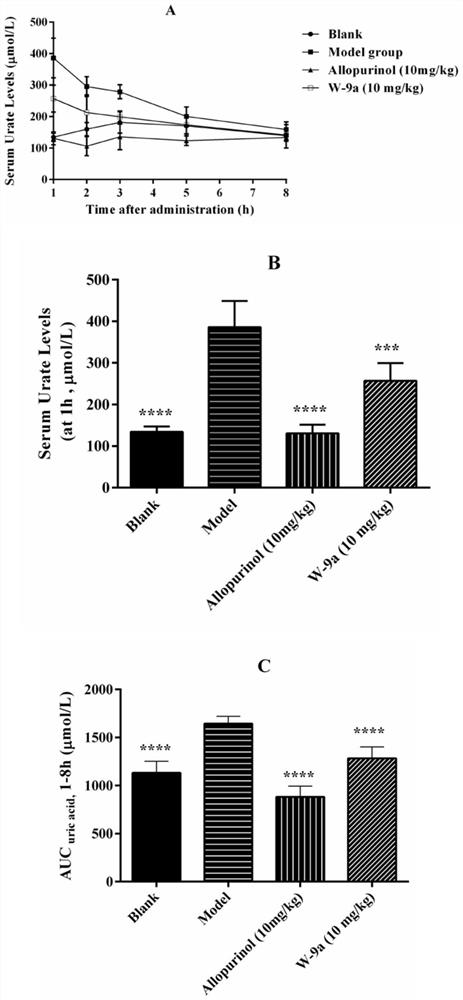
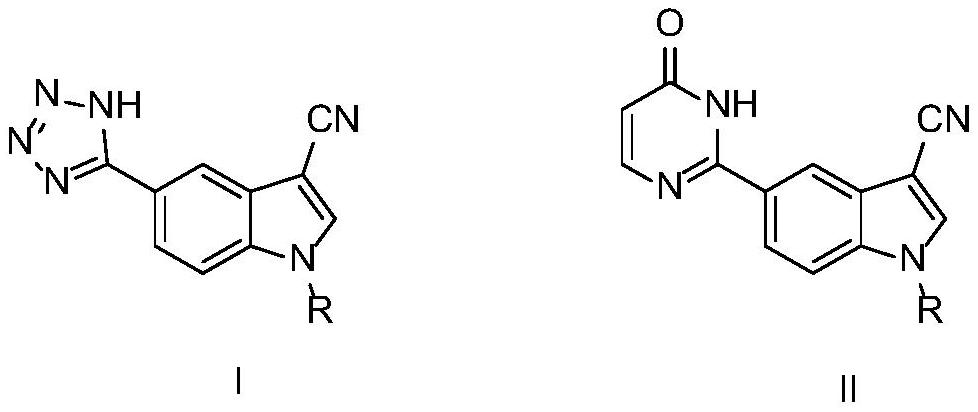
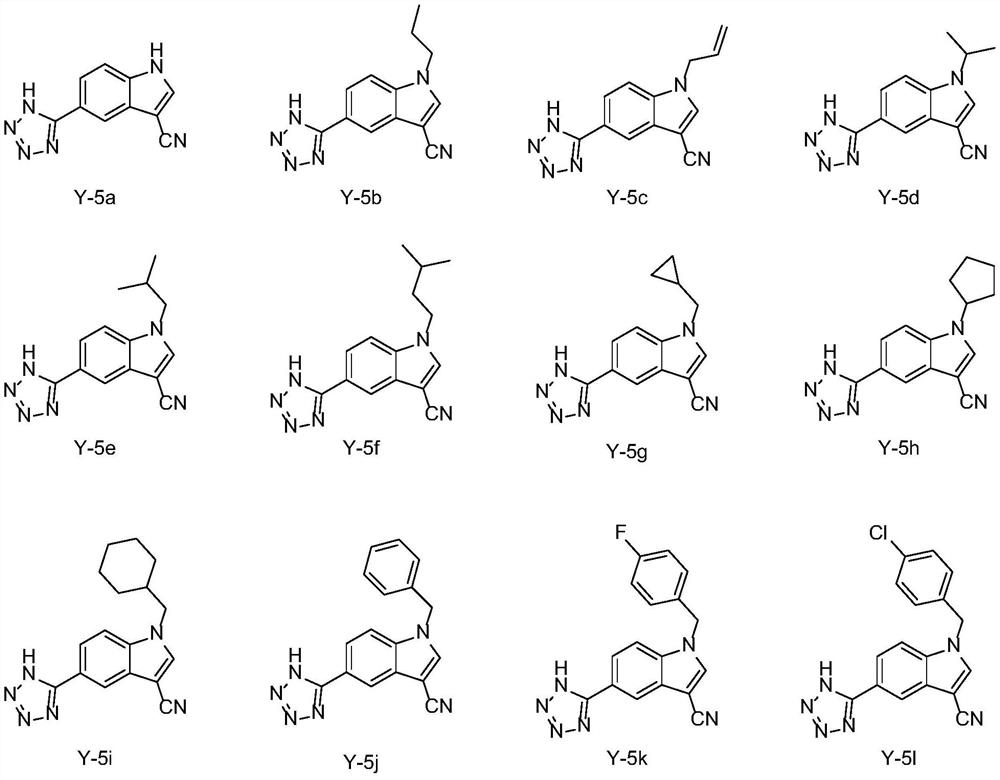
1-alkyl-5-tetrazolyl/pyrimidinone-1H-indole-3-formonitrile compounds as well as preparation method and application thereof
A pyrimidinone-based and tetrazolyl-based technology, which is applied in the application field of treating and/or preventing hyperuricemia and gout, can solve the problems of undiscovered preparation methods and uses of formic nitrile compounds, and achieves reduction of serum uric acid. level, the effect of good research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Preparation of 5-(1H-tetrazol-5-yl)-1H-indole-3-carbonitrile (Y-5a)
[0069] Preparation of 5-cyano-1H-indole-3-carbaldehyde (Y-3a): 30.0 g (0.211 mol) of 5-cyano indole was added to 200 mL of DMF, and slowly added dropwise under stirring at room temperature Phosphorus 15.0g (0.098mol), after the dropwise addition, mechanically stirred at 75°C for 8h. TLC monitoring, after the reaction is completed, slowly pour 200mL of water into the reaction solution, adjust the pH to 7-8 with 2M sodium hydroxide aqueous solution, continue mechanical stirring at 75°C for 30min, a large amount of yellow solid precipitates, filter with suction, and use 200mL of the filter cake Washed with water three times, air-dried at 50°C for 10 hours to obtain 39.0 g of yellow solid, yield 94.2%, Mp 144.2°C-145.6°C. 1 H NMR (400MHz, DMSO-d 6 )δ11.48(s, 1H), 8.31(d, J=1.7Hz, 1H), 7.81(dd, J=8.5, 1.7Hz, 1H), 7.62(d, J=8.5Hz, 1H), 7.51( t,J=2.8Hz,1H),6.63(s,1H).
[0070] Preparation of 5...
Embodiment 2
[0072]Example 2: Preparation of 1-n-propyl-5-(1H-tetrazol-5-yl)-1H-indole-3-carbonitrile (Y-5b)
[0073] Preparation of 5-cyano-1H-indole-3-carbaldehyde (Y-3a): In a 1000mL three-neck flask, add 30.0g (0.211mol) 5-cyanoindole, 200.0mL DMF, mechanically Stir for 10 min. Slowly drop 15.0 g (0.098 mol) of phosphorus oxychloride into the above reaction solution, and after the drop is completed, mechanically stir at 75° C. for 8 h. After the reaction is complete, slowly pour 200mL of water into the above reaction solution, adjust the pH to 7-8 with 2M aqueous sodium hydroxide solution, continue to stir at 75°C for 45 minutes, a yellow solid precipitates, suction filters, washes with water, and dries to obtain yellow Solid 39.5g, yield: 94.6%, mp: 144.2-145.6°C. MS(ESI) m / z: 169.0[M-H] - ; 1 H NMR (400MHz, DMSO-d 6 )δ11.48(s, 1H), 8.31(d, J=1.7Hz, 1H), 7.81(dd, J=8.5, 1.7Hz, 1H), 7.62(d, J=8.5Hz, 1H), 7.51( t,J=2.8Hz,1H),6.63(s,1H).
[0074] Preparation of 1-n-propyl-5-cyano-1...
Embodiment 3
[0077] Example 3: Preparation of 1-allyl-5-(1H-tetrazol-5-yl)-1H-indole-3-carbonitrile (Y-5c)
[0078] Preparation of 5-cyano-1H-indole-3-carbaldehyde (Y-3a): In a 1000mL three-neck flask, add 30.0g (0.211mol) 5-cyanoindole, 200.0mL DMF, mechanically Stir for 10 min. Slowly drop 15.0 g (0.098 mol) of phosphorus oxychloride into the above reaction solution, and after the drop is completed, mechanically stir at 75° C. for 8 h. After the reaction is completed, slowly pour 200mL of water into the above reaction solution, adjust the pH to 7-8 with 2M sodium hydroxide aqueous solution, continue stirring at 75°C for 30 minutes, a yellow solid precipitates, suction filters, washes with water, and dries to obtain yellow Solid 38.0g, yield: 93.4%, mp: 144.2-145.6°C. MS (ESI) m / z: 169.0 [M-H] - ; 1 H NMR (400MHz, DMSO-d 6 )δ11.48(s, 1H), 8.31(d, J=1.7Hz, 1H), 7.81(dd, J=8.5, 1.7Hz, 1H), 7.62(d, J=8.5Hz, 1H), 7.51( t,J=2.8Hz,1H),6.63(s,1H).
[0079] Preparation of 1-allyl-5-cyano-1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com