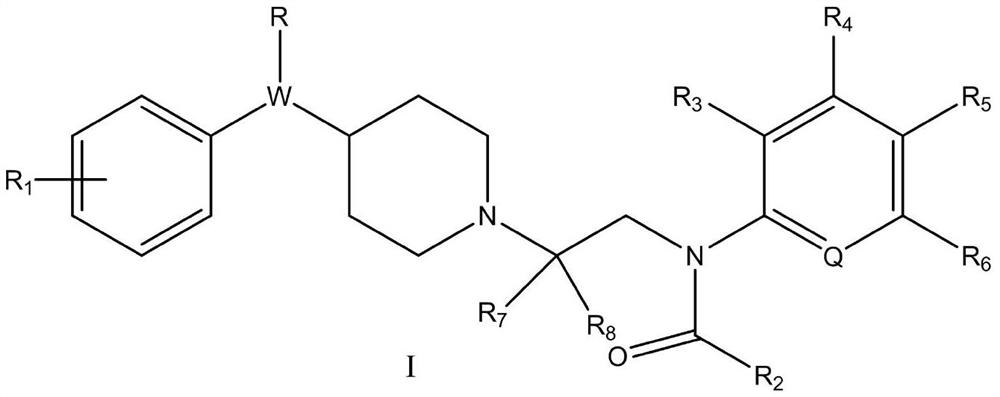
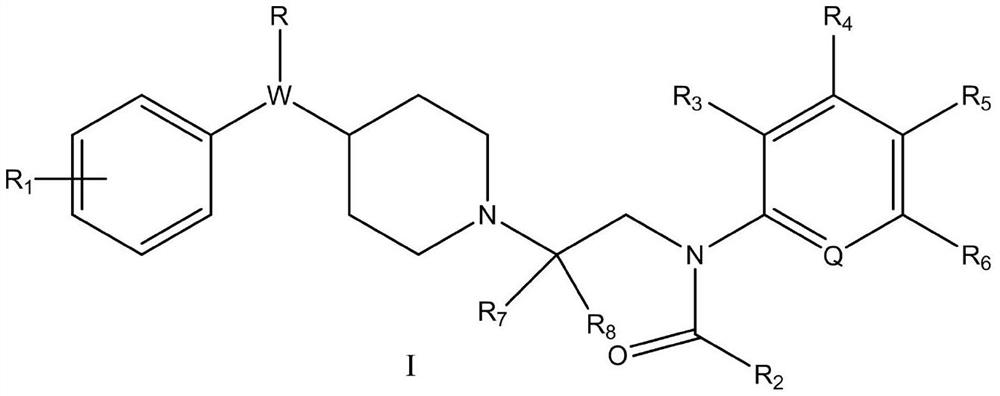
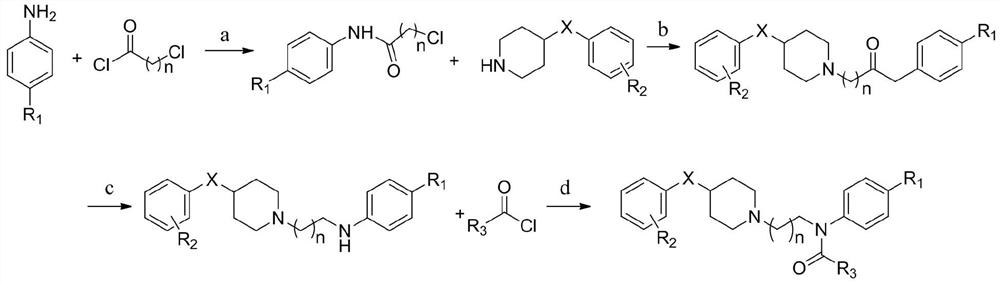
Piperidine amide derivative, and pharmaceutical composition and application thereof
A piperidine and compound technology, applied in the field of medicinal chemistry, can solve the problems of low response rate, no evaluation of in vivo analgesic activity, strong side effects, etc., and achieve the effect of good analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: N-(2-(4-benzylpiperidin-1-yl)ethyl)-N-phenylacetamide (target compound 1)
[0092] Reaction 1
[0093]
[0094] general method of synthesis
[0095] 1) Synthesis of 2-chloro-N-phenylacetamide (intermediate 1):
[0096]
[0097] With 1g (10.74mmol) aniline, 2.969g (21.48mmol) K 2 CO 3 Dissolve in 10mL acetone, place in an ice bath and stir to 0°C. 1.456 g (12.89 mmol) of chloroacetyl chloride was slowly added dropwise, and stirred at room temperature for 4 hours. Add water to quench after the reaction, extract with EA, wash with dilute NaOH solution and saturated brine respectively, add anhydrous sodium sulfate to stir and dry, distill under reduced pressure to remove the solvent, add appropriate amount of absolute ethanol and petroleum ether to heat to dissolve, cool Crystallized, filtered and washed the filter cake with petroleum ether, and dried to obtain a white solid with a yield of about 54.7%.
[0098] 2) Synthesis of 2-(4-benzylpiperidin-...
Embodiment 2
[0108] Example 2: N-(2-(4-benzylpiperidin-1-yl)ethyl)-N-(4fluorophenyl)acetamide (target compound 2)
[0109] The difference from Example 1 is only that in the synthesis step 1), aniline is replaced by p-fluoroaniline, and other steps are basically the same, so they will not be repeated here.
[0110] 1 H NMR (400MHz, CDCl 3 )δ7.26(t,J=7.4Hz,2H),7.23–7.15(m,3H),7.15–7.04(m,4H),3.79(t,J=7.0Hz,2H),2.82(d,J =11.4Hz, 2H), 2.51(d, J=7.1Hz, 2H), 2.43(t, J=7.0Hz, 2H), 1.92–1.84(m, 2H), 1.81(s, 3H), 1.58(d ,J=13.1Hz,2H),1.48–1.43(m,1H),1.29–1.16(m,2H).MS(ESI)m / z=355.2([M+H] + )
Embodiment 3
[0111] Example 3: N-(2-(4-benzylpiperidin-1-yl)ethyl)-N-(4methylphenyl)acetamide (target compound 3)
[0112] The difference from Example 1 is only that in the synthesis step 1), the aniline is replaced by p-methylaniline, and the other steps are basically the same, and will not be repeated here.
[0113] 1 H NMR (400MHz, CDCl 3 )δ7.29–7.24(m,2H),7.20–7.15(m,3H),7.12(dd,J=5.1,3.1Hz,2H),7.09–7.05(m,2H),3.86–3.76(m, 2H), 2.85(d, J=11.3Hz, 2H), 2.51(d, J=7.1Hz, 2H), 2.47–2.42(m, 2H), 2.37(s, 3H), 1.90(t, J=11.1 Hz,2H),1.81(s,3H),1.58(d,J=13.1Hz,2H),1.53–1.42(m,1H),1.30–1.18(m,2H).MS(ESI)m / z= 351.2([M+H] + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com