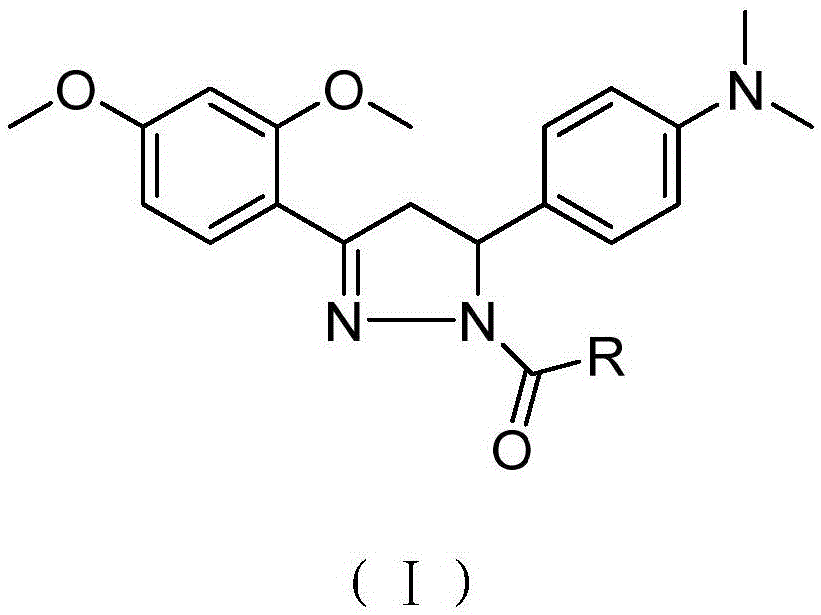
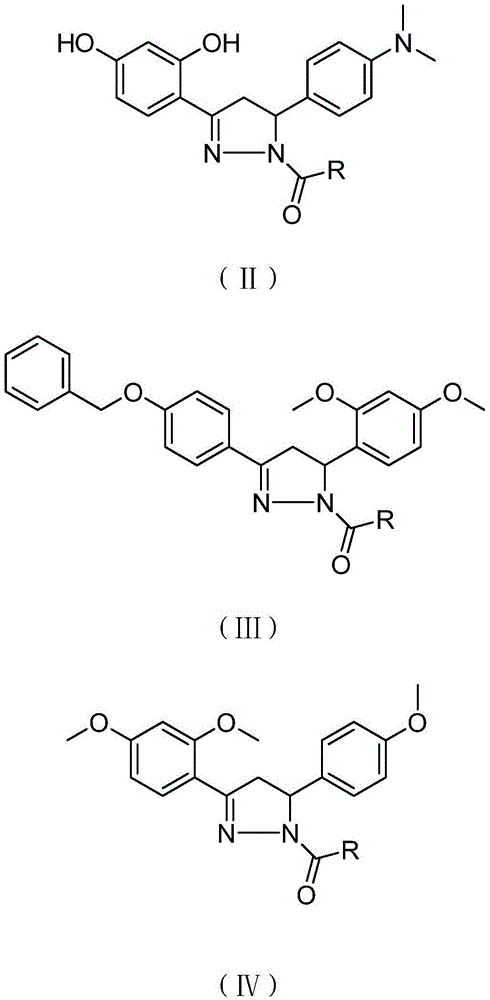
A compound with analgesic effect and preparation method thereof
A technology of compounds and target compounds, applied in the field of compounds with analgesic effect and their preparation, can solve the problems of clinical reference restrictions, addiction and dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Example 1 Compound (I-1)
[0036]
[0037] (1) Synthesis of p-dimethylaminobenzaldehyde
[0038]In a 100mL three-necked flask, add 4.9g (0.032mol) of phosphorus oxychloride, and slowly add 2.5g (0.031mol) of DMF dropwise under ice bath. N-dimethylaniline, after dropping, move the reaction solution to a boiling water bath for 2-4 hours (monitor the end point of the reaction with thin-layer chromatography), after the reaction is completed, pour the reaction solution into 20 mL of ice water, and use 30% hydrogen The sodium oxide solution was used to adjust the pH to 4-4.5, left to stand for crystallization for 24 hours, and suction filtered to obtain a light yellow or nearly colorless solid, which was recrystallized with 30 mL of ethanol-water (1:2.5) to obtain the product p-dimethylaminobenzaldehyde.
[0039]
[0040] (2) Synthesis of 2,4-methoxyacetophenone
[0041] Into a 100mL three-necked flask equipped with a condenser, a stirrer, and a thermom...
Embodiment 2
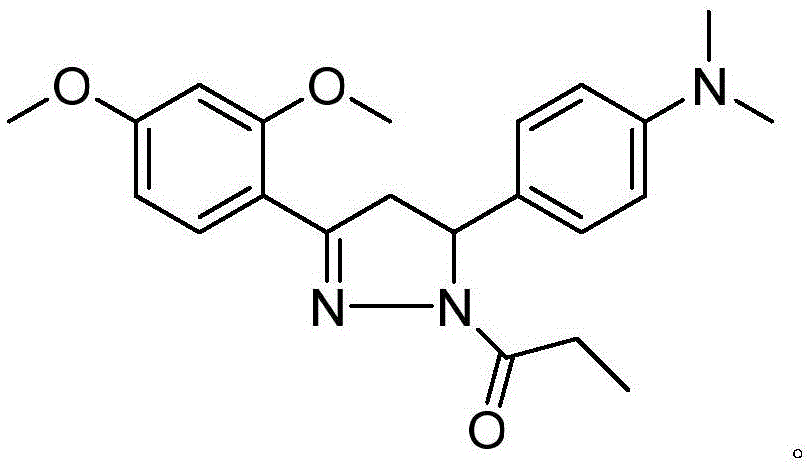
[0049] The synthesis of embodiment 2 compound (I-2)
[0050]
[0051] Add 15mL of propionic acid and 0.7g (0.014mol) of 80% hydrazine hydrate to a 100mL three-necked flask, stir and heat for 0.5h, then add 1.09g (0.0035mol) of 4-dimethylamino-2,4-dimethoxy Chichalcone, stirred and heated to 80°C for 6-8 hours (monitor the end point of the reaction with thin-layer chromatography), after the reaction is complete, adjust the pH of the obtained solution to 7-8 with dilute sodium hydroxide solution, and then dilute the solution with 20mL×3 Methyl chloride was extracted three times, and the obtained organic phase was washed three times with 20 mL×3 water, dried over anhydrous sodium sulfate, filtered, and the solvent was removed to obtain a brown oil. The crude product was separated with a silica gel column (10 g of 200-300 mesh silica gel was loaded into a silica gel column for 1 g of sample). The eluent volume ratio is v (acetone): v (petroleum ether) = 7:3. A yellow solid wa...
Embodiment 3
[0052] The synthesis of embodiment 3 compound (I-3)
[0053]
[0054] Add 15 mL of butyric acid and 0.7 g (0.014 mol) of 80% hydrazine hydrate to a 100 mL three-necked flask, stir and heat for 0.5 h, then add 1.09 g (0.0035 mol) of 4-dimethylamino-2,4-dimethoxy Chilchalcone, stirred and heated to 80°C for 5-7 hours (monitor the end point of the reaction with thin-layer chromatography), after the reaction is complete, adjust the pH of the obtained solution to 7-8 with dilute sodium hydroxide solution, and then use 20ml×3 Methyl chloride was extracted three times, and the obtained organic phase was washed three times with 20ml×3 water, dried over anhydrous sodium sulfate, filtered, and the solvent was removed to obtain a brown oil. The crude product was separated with a silica gel column (10 g of 200-300 mesh silica gel was loaded into a silica gel column for 1 g of sample). The eluent volume ratio is v (acetone): v (petroleum ether) = 7:3. A yellow oil was obtained, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com