Application of pharmaceutical composition preparation in preparation of medicine for treating 2019-nCoV infected critical patient
A 2019-ncov and drug technology, applied in the field of medicine, can solve the problems of safety pending clinical research demonstration, complexity, time-consuming, etc., and achieve the effect of extensive pharmaceutical value, strong safety and good efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Construction of ACE2-His MSC
[0046] This embodiment builds ACE2-His MSC, comprises the following steps:
[0047] Step 1: Construction of lentivirus
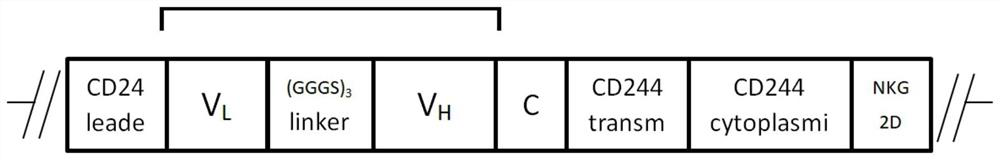
[0048] Step 1.1: constructing the recombinant vector ACE2-his;
[0049] Step 1.2: Construct the lentiviral vector Plenti-ACE2-his;
[0050] Step 1.3: Lentiviral packaging;
[0051] Step 2: MSC Cell Culture Expansion
[0052] Take the fresh umbilical cord of a healthy newborn, wash the umbilical cord with PBS, remove the blood vessels, peel off the Wahrenheit tissue, and fully cut the remaining tissue to 1mm 3 size, add α-MEM medium, at 37°C, 5% CO 2 Cultivate in an incubator, and after the cells are confluent, digest and passage with 0.25% trypsin; add PBS to adjust the cell concentration to 1×10 6 / mL to obtain MSC cells.
[0053] Step 3: Infect the MSC cells obtained in step 2 with the lentivirus obtained in step 1, and then culture;
[0054] Step 4: Antibiotic screening to obtain stable cell lines; ...
Embodiment 2
[0056] Example 2: Construction of CAR-NK antiHis
[0057] The construction of CAR-NK antiHis in this embodiment includes the following steps:
[0058] Step 1: Construction of lentivirus
[0059] Step 1.1: Construction of the recombinant vector CAR-NK-anti-his;
[0060] Step 1.2: Construction of lentiviral vector Plenti-CAR-NK-anti-his;
[0061] Step 1.3: Lentiviral packaging;
[0062] Step 2: NK cell culture expansion
[0063] 200 mL of peripheral blood was drawn, NK cells were sorted, residual red blood cells were removed, and NK cells were expanded and cultured to obtain NK cells.
[0064] Step 3: Infect the NK cells obtained in step 2 with the lentivirus obtained in step 1, and then culture;
[0065] Step 4: Anti-his CAR-NK cells are collected and stored;
[0066] Step 5: incubation and detection of fluorescently labeled his antigen;
[0067] Step 6: Detection of anti-his CAR expression by RT-PCR.
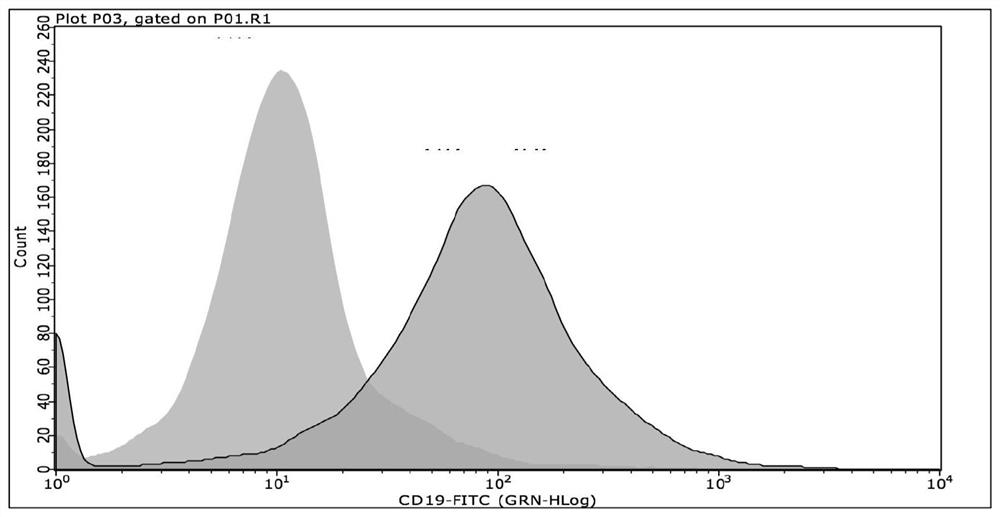
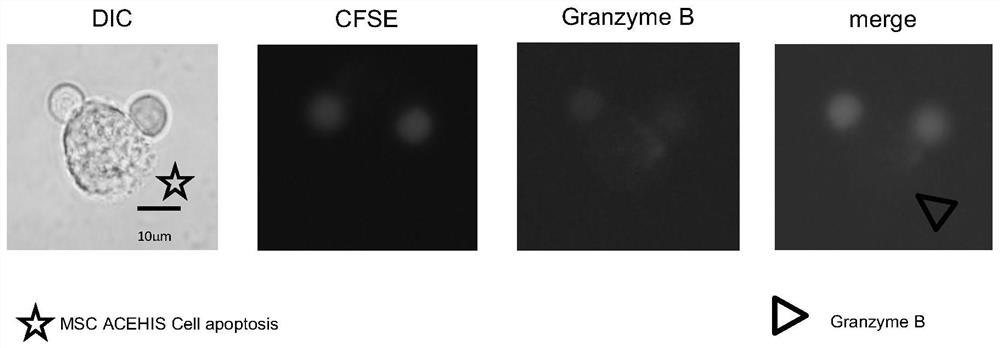
[0068] Experimental results
[0069] Such as figure 1 As shown, N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com