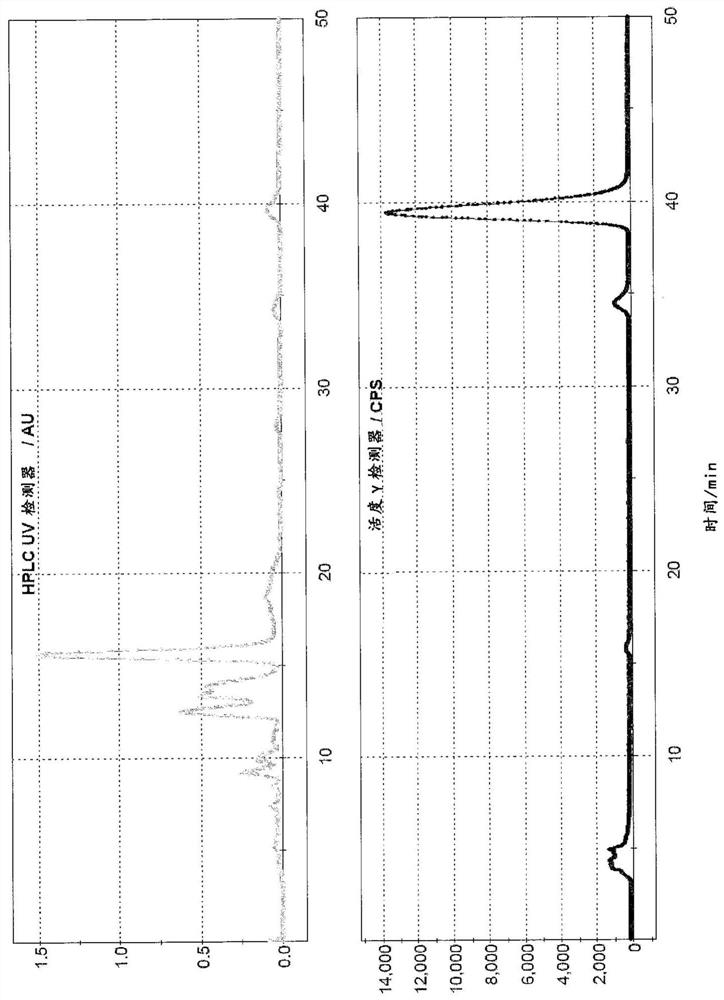
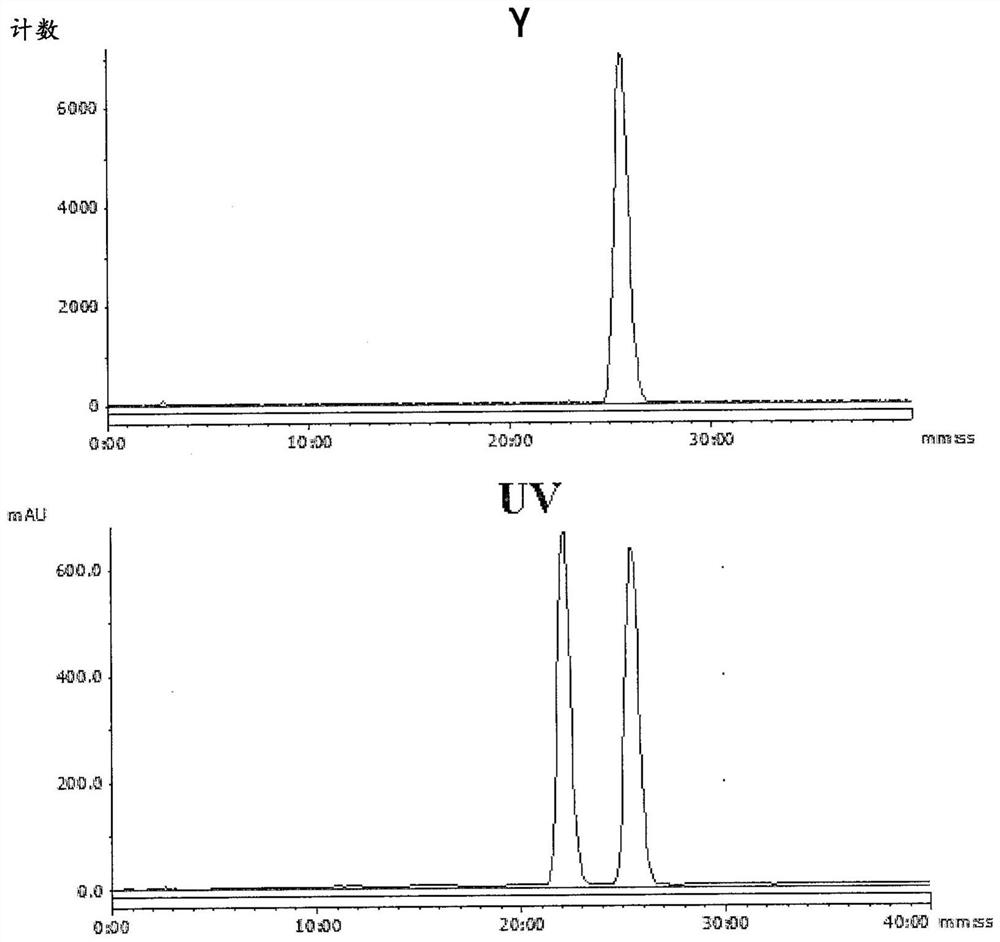
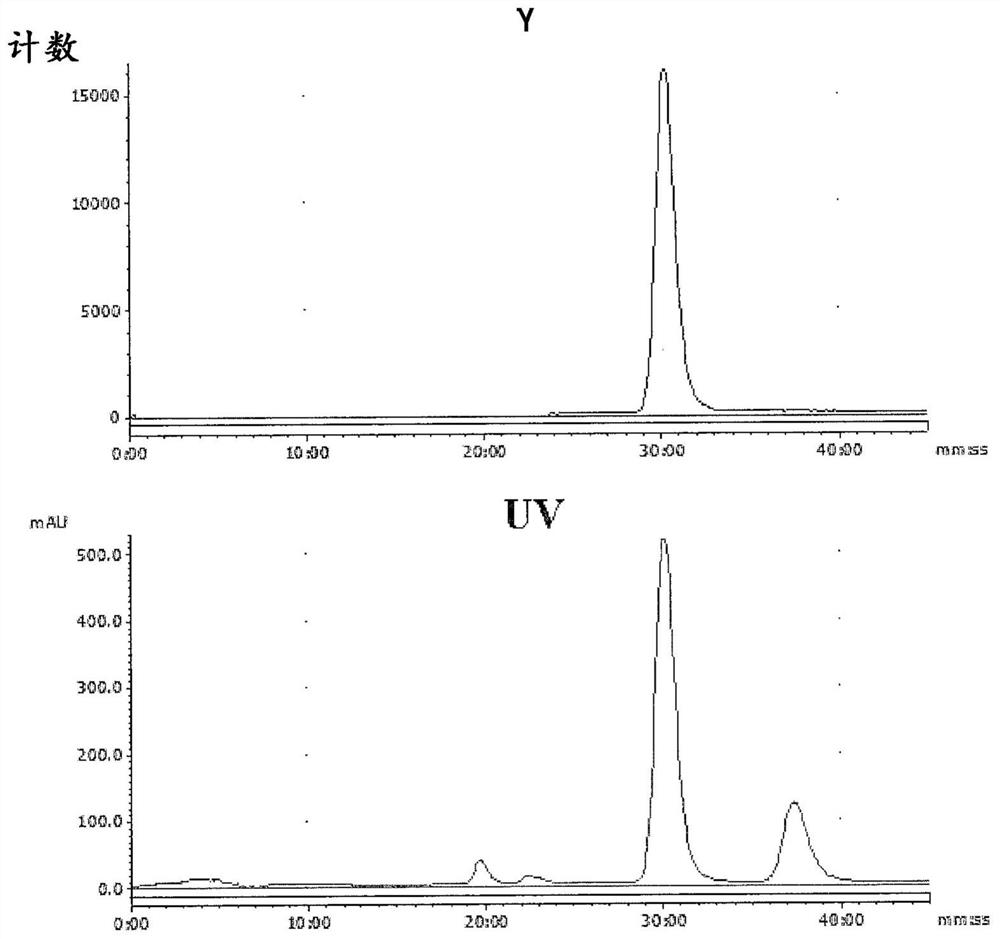
Radioligands for imaging lpa1 receptor
A technology of radioactive labeling and receptors, applied in the direction of radioactive carriers, radioactive preparations in vivo, and instruments for radiological diagnosis, etc., which can solve problems such as undesired side effects, limited treatment options, and no cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1. (1S, 3S)-3-((6-(5-(hydroxymethyl)-1-methyl-1H-1,2,3-triazol-4-yl)-2-methyl Pyridin-3-yl)oxy)cyclohexane-1-carboxylic acid methyl ester
[0123]
[0124] The title compound was prepared according to the synthetic sequence described for the preparation of the corresponding isopropyl ester (Example IE) or ethyl ester (Example 10F) from patent application US 2017 / 0360759. The starting material used to prepare the title compound was (1S,3R)-methyl 3-hydroxycyclohexane-1-carboxylate (instead of the corresponding cyclohexane isopropyl or ethyl ester).
Embodiment 2
[0125] Example 2. (1R,3S)-3-((6-(5-(hydroxymethyl)-1-methyl-1H-1,2,3-triazol-4-yl)-2-methyl Pyridin-3-yl)oxy)cyclohexane-1-carboxylic acid methyl ester
[0126]
[0127] To a clear solution of the compound of Example 1 (1.95 g, 5.41 mmol) in MeOH (38.6 mL) was added 5.4M NaOMe solution (4.0 mL, 21.64 mmol). The reaction was heated at 60 °C for 6 h and then cooled to room temperature. The reaction was placed in an ice bath, neutralized with 1 N HCl, and then partially concentrated to remove MeOH. The resulting cloudy mixture was partitioned at 1.0MK 2 HPO 4 with EtOAc, and the layers were separated. The aqueous layer was extracted with EtOAc (1x). The organic layers were combined and washed with brine, dried (Na 2 SO 4 ), filtered and concentrated to give a white solid, weighing 1.66 g. Purification by chiral SFC Prep (column: Chiralpak IA, 21x250mm, 5 microns; mobile phase: 20% MeOH / 80% CO 2 ; flow conditions: 85 mL / min, 150 bar, 40° C.; detector wavelength: 254 nm...
Embodiment 3
[0128] Example 3. (1S,3S)-3-((2-methyl-6-(1-methyl-5-((((4-nitrophenoxy)carbonyl)oxy)methyl)- 1H-1,2,3-triazol-4-yl)pyridin-3-yl)oxy)cyclohexane-1-carboxylic acid methyl ester
[0129]
[0130] To a solution of the compound of Example 1 (970 mg, 2.69 mmol) and pyridine (1.09 mL, 13.5 mmol) in DCM (17.9 mL) at 0 °C was added dropwise over 1 h 4-nitrophenyl chloroformate (1.09 g, 5.38 mmol) in DCM (3 mL). The reaction was then allowed to warm to room temperature and stirred at room temperature for 20 h, then concentrated in vacuo to give a solid. A minimal amount of DCM was added to give a suspension and the solid (pyridine hydrochloride) was removed by filtration. The filtrate was concentrated in vacuo. The residue was chromatographed (120 g SiO 2 column; continuous gradient from 0-50% EtOAc in hexanes) to give the title compound (1.12 g, 79% yield) as a pale yellow solid. LCMS,[M+H] + = 526.3. 1 H NMR (500MHz, CDCl 3 )δ8.33-8.28(m,2H),8.03(d,J=8.5Hz,1H),7.43-7.38(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com