Multimeric hybrid fc proteins for replacement of ivig
A protein and duplex technology, applied in hybrid immunoglobulin, anti-animal/human immunoglobulin, immunoglobulin, etc., can solve the problem of short serum half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: General Methods and Materials
[0090] Using standard laboratory techniques (e.g. Green and Sambrook (Molecular Cloning, A Laboratory Manual, Fourth Edition, 2012, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY), Greenfield (Antibodies, A Laboratory Manual, Second Edition, 2014, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY), Kostelny et al. (Int. J. Cancer 93:556-565, 2001), Cole et al. (J. Immunol. 159:3613-3621, 1997), and Tsurushita Manipulation of recombinant DNA and expression, purification and characterization of recombinant proteins were performed as described by et al. (Methods 36:69-83, 2005).
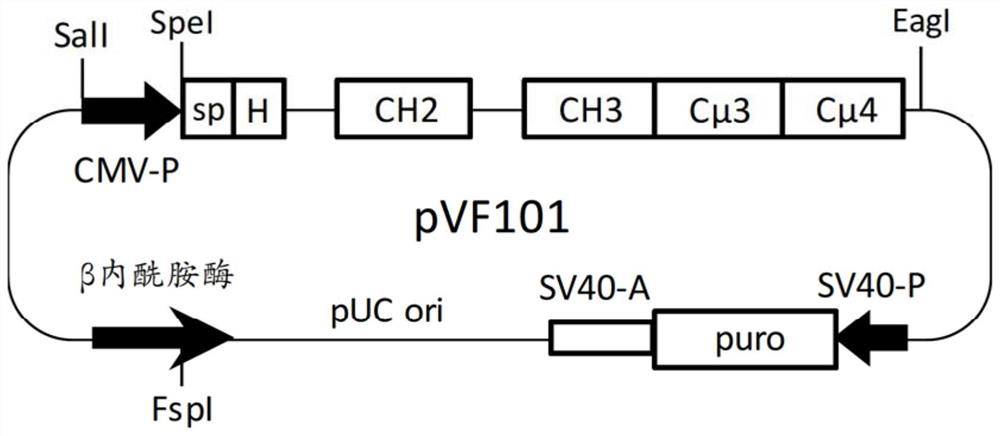
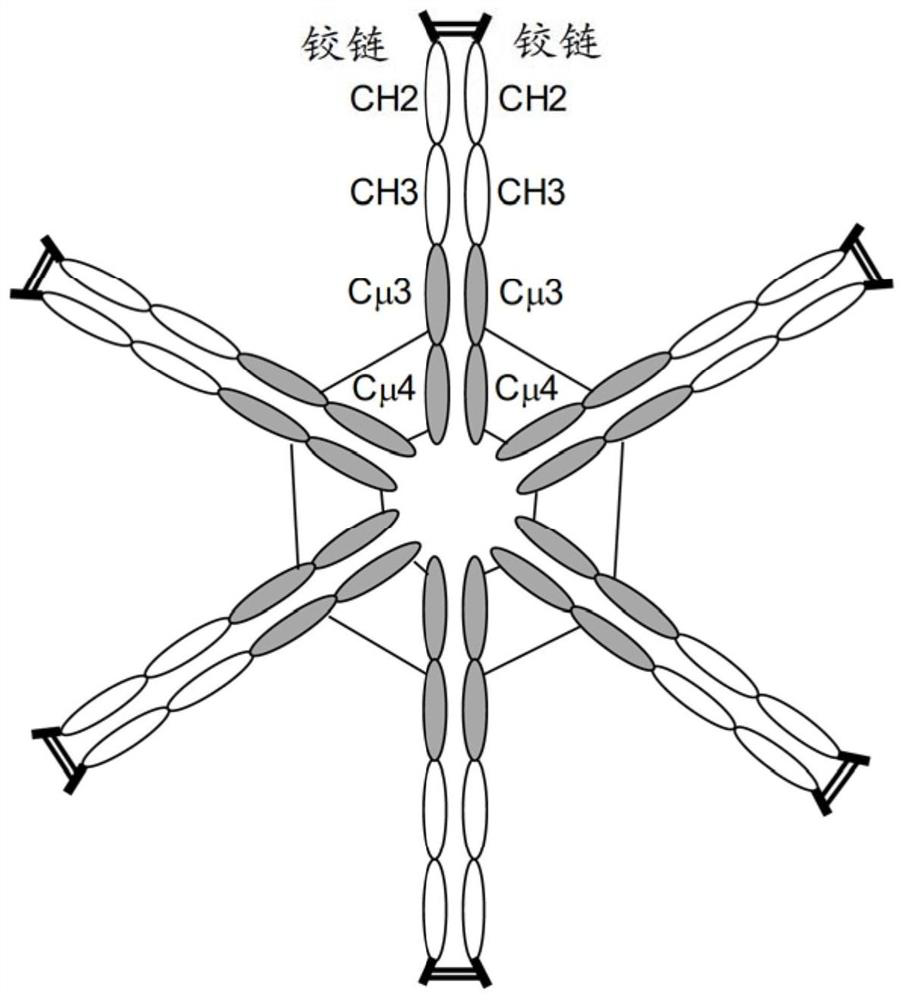
[0091] Mammalian expression vector pVF101 ( figure 1 ) was designed to generate a multimeric hybrid Fc protein comprising an artificial signal peptide (sp) from N-terminus to C-terminus, a hinge, the CH2 and CH3 regions of the human IgG1 isotype, followed by human Cμ3 and Cμ4 region, containing the following genetic components. ...
Embodiment 2
[0100] Example 2: Expression and purification of multimer hybrid Fc protein
[0101] The expression vectors pVF102 and pVF103 were introduced into the chromosome of Chinese hamster ovary cell line CHO-K1 to obtain cell lines that stably produce LS41K-Fc.S and LS41K-Fc.SL, respectively. CHO-K1 cells were grown in SFM4CHO medium (GE Healthcare, Chicago, IL) at 37°C in 7.5% CO 2 Grow in an incubator. It was stably transfected into CHO-K1 by electroporation. Before transfection, each expression vector was linearized using FspI. In a typical experiment, approximately 10 7 Each cell was transfected with 20 μg of linearized plasmid, suspended in SFM4CHO medium, and seeded into several 96-well plates after appropriate dilution of cells. After 48 hours, puromycin was added to isolate stable transfectants. Approximately twelve days after initiation of selection, culture supernatants from transfectants were assayed for antibody production.
[0102] Expression of LS41K-Fc.S and LS41...
Embodiment 3
[0104] Example 3: Analysis of Pharmacokinetics (PK) and Pharmacodynamics (PD) of LS41K-Fc.S in Mice
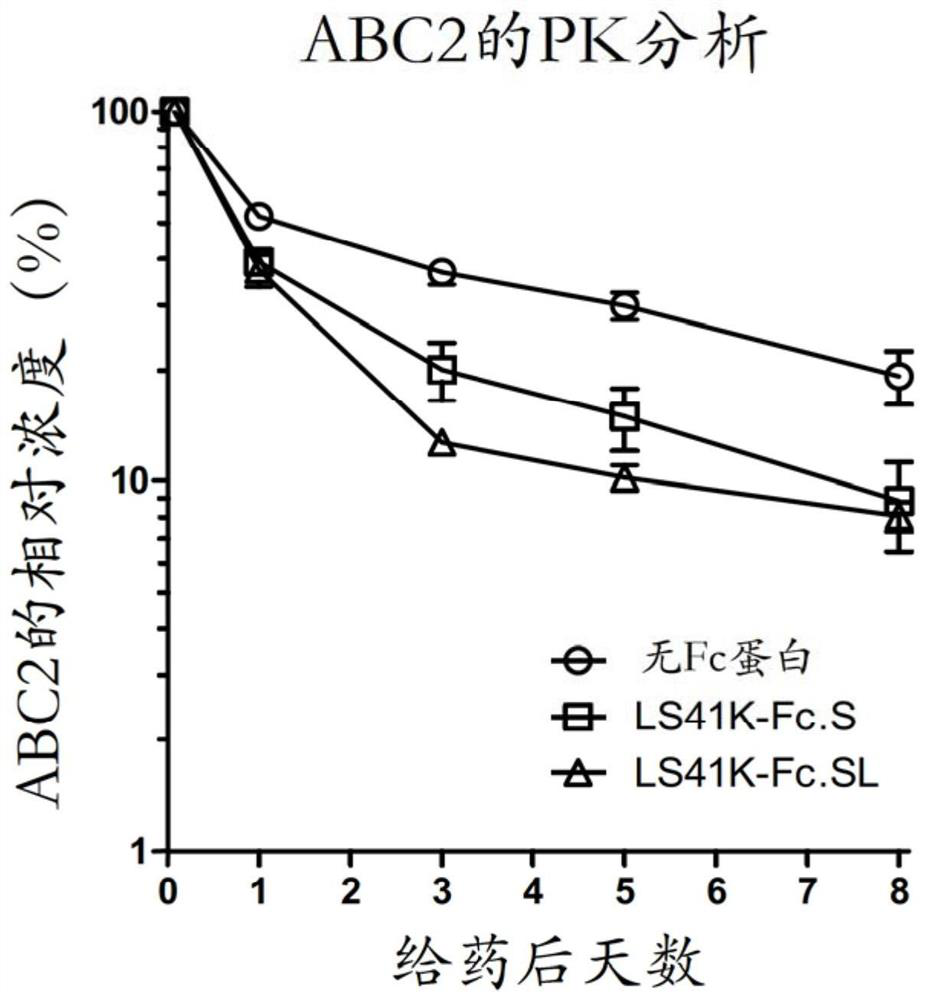
[0105] Fifty (50) μg of mouse monoclonal anti-human CD122 IgG1 antibody ABC2 was intracardially administered to each group in the presence and absence of 400 μg LS41K-Fc.S in 50 μl of PBS (groups A and B, respectively) in three Balb / c mice. On the day before dosing (Day -1), two hours after dosing (2HR), one day (Day 1), three days (Day 3), five days (Day 5) and eight days (Day 8) Serum samples were then collected from these mice.
[0106] ABC2 concentrations in serum samples were measured by ELISA as described above. ABC2 concentrations at each time point (Day 1, Day 3, Day 5, and Day 8) were normalized to the concentration in the 2HR sample of each mouse. The data is plotted on image 3 middle. Mean relative concentrations of ABC2 (ABC2 only) in Group A were 100% (2HR), 52.0% (Day 1), 36.7% (Day 3), 29.9% (Day 5) and 19.3% (Day 8) . In contrast, the mean percentage co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com