Preparation method of polysubstituted cyclobutane compound
A compound, cyclobutane technology, applied in the field of preparation of multi-substituted cyclobutane compounds, to achieve the effect of easy large-scale production, high reactivity, and rich substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of cyclobutane compound 3a, reaction equation is as follows:
[0032]
[0033] In the reaction flask, add compound 1a (100mmol), compound 2a (500mmol), Ir (ppy) 3 (1mmol), Rh 2 (OAc) 4 (5mmol), 1,2-dichloroethane (DCE, 500mL), irradiated under blue LED lamp for 12h. After the reaction was completed, the reaction solvent was removed by a rotary evaporator to obtain a crude product, which was separated by column chromatography to obtain the target product 3a with a yield of 80%.
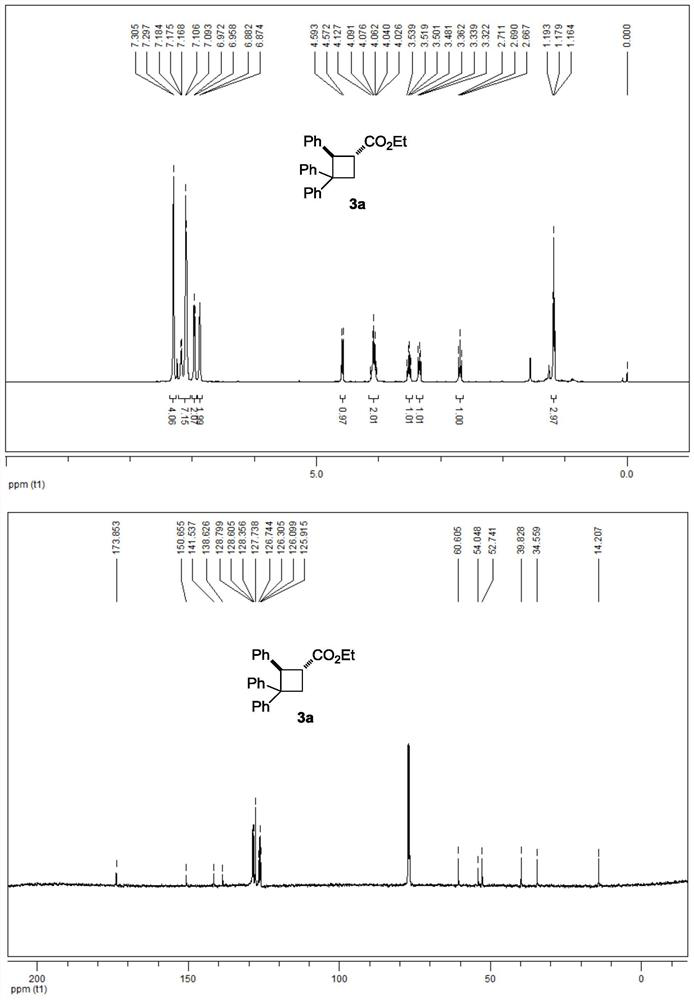
[0034] 3a NMR data:
[0035] 1 H NMR (500MHz, CDCl 3 ): δ7.30(d, J=4.0Hz, 4H), 7.18-7.09(m, 7H), 6.96(d, J=7.0Hz, 2H), 6.88(d, J=4.0Hz, 2H), 4.58 (d,J=10.5Hz,1H),4.13-4.03(m,2H),3.51(q,J=10.0Hz,1H),3.36-3.32(m,1H),2.69(t,J=11.0Hz, 1H), 1.18(t, J=7.0Hz, 3H)ppm.
[0036] 13 C NMR (125MHz, CDCl 3 ): δ173.9, 150.7, 141.5, 138.6, 128.8, 128.6, 128.4, 127.7, 126.7, 126.3, 126.1, 125.9, 60.6, 54.0, 52.7, 39.8, 34.6, 14.2ppm.
Embodiment 2
[0038] The preparation of cyclobutane compound 3b, the reaction equation is as follows:
[0039]
[0040] In the reaction flask, add compound 1a (100mmol), compound 2b (500mmol), Ir (ppy) 3 (1mmol), Rh 2 (OOc) 4 (5mmol), 1,2-dichloroethane (DCE, 500mL), irradiated under blue LED lamp for 12h. After the reaction, the reaction solvent was removed using a rotary evaporator to obtain a crude product, which was separated by column chromatography to obtain the target product 3b with a yield of 60% (3:1d.r.).
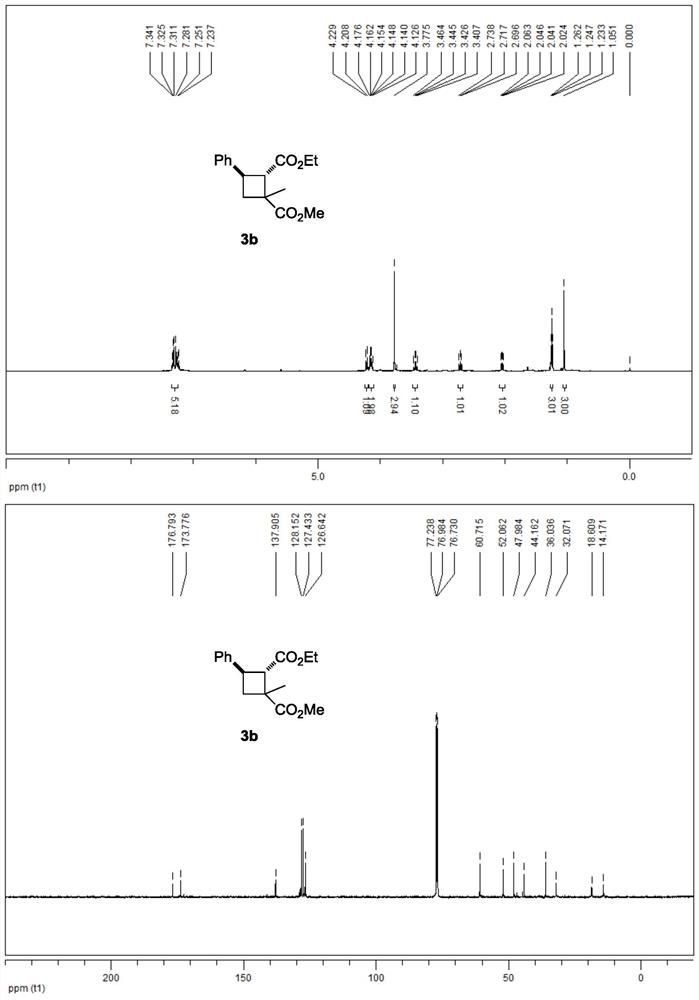
[0041] 3b NMR data are as follows:
[0042] 1 H NMR (500MHz, CDCl 3 ):δ7.34-7.23(m,5H),4.22(d,J=10.5Hz,1H),4.18-4.13(m,2H),3.78(s,3H),3.44(q,J=9.5Hz, 1H), 2.72(t, J=10.5Hz, 1H), 2.04(dd, J=8.5, 11.0Hz, 1H), 1.25(t, J=7.5Hz, 3H), 1.05(s, 3H)ppm.
[0043] 13 C NMR (125MHz, CDCl 3 ): δ176.8, 173.8, 137.9, 128.2, 127.4, 126.6, 60.7, 52.1, 48.0, 44.2, 36.0, 32.1, 18.6, 14.2ppm.
Embodiment 3
[0045] The preparation of cyclobutane compound 3c, reaction equation is as follows:
[0046]
[0047] In the reaction flask, add compound 1a (100mmol), compound 2c (500mmol), Ir (ppy) 3 (1mmol), Rh 2 (OAc) 4 (5mmol), 1,2-dichloroethane (DCE, 500mL), irradiated under blue LED lamp for 12h. After the reaction, the reaction solvent was removed using a rotary evaporator to obtain a crude product, which was separated by column chromatography to obtain the target product 3c with a yield of 85% (10:1d.r.).
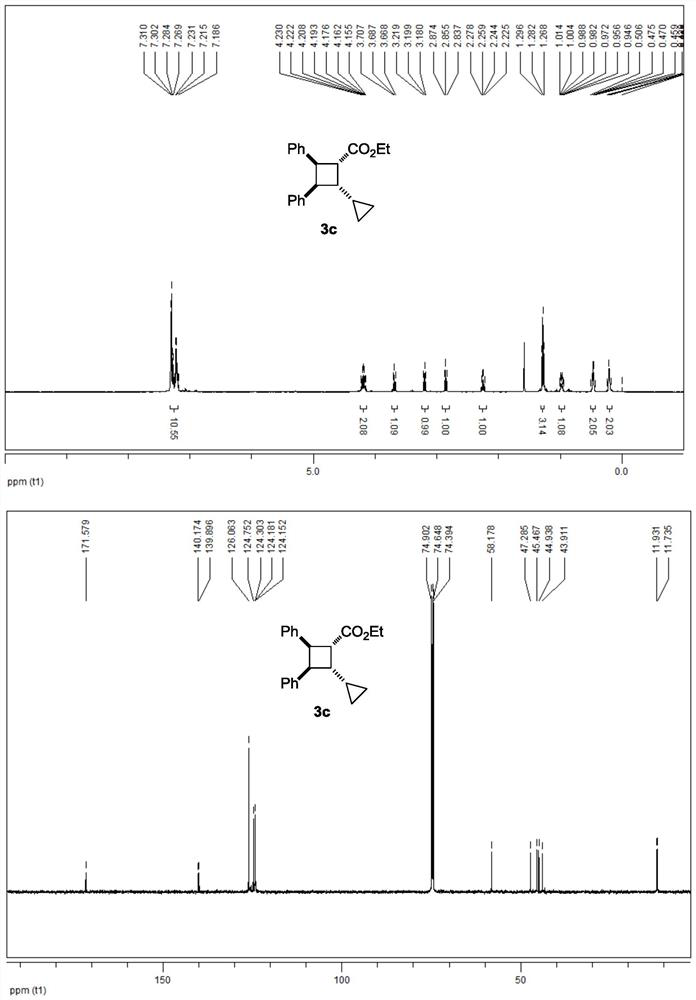
[0048] 3c NMR data are as follows:
[0049] 1 H NMR (500MHz, CDCl 3 ):δ7.31-7.19(m,10H),4.23-4.16(m,2H),3.69(t,J=10.0Hz,1H),3.20(t,J=10.0Hz,1H),2.86(t, J=9.5Hz, 1H), 2.25(q, J=9.5Hz, 1H), 1.28(t, J=7.0Hz, 3H), 1.01-0.95(m, 1H), 0.51-0.43(m, 2H), 0.24-0.18(m,2H)ppm.
[0050] 13 C NMR (125MHz, CDCl 3 ): δ171.6, 140.2, 139.9, 126.1, 124.8, 124.3, 124.2, 124.2, 58.2, 47.3, 45.5, 44.9, 43.9, 11.9, 11.7ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com