A kind of preparation method of polysubstituted cyclobutane compound
A compound, cyclobutane technology, which is applied in the field of preparation of polysubstituted cyclobutane compounds, and achieves the effects of rich substrate range, easy large-scale production and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of cyclobutane compound 3a, the reaction equation is as follows:
[0032]
[0033] Add compound 1a (100 mmol), compound 2a (500 mmol), Ir(ppy) to the reaction flask 3 (1mmol), Rh 2 (OAc) 4 (5 mmol), 1,2-dichloroethane (DCE, 500 mL), irradiated under a blue LED light for 12 h. After the reaction, the reaction solvent was removed using a rotary evaporator to obtain a crude product. The crude product was separated by column chromatography to obtain the target product 3a with a yield of 80%.
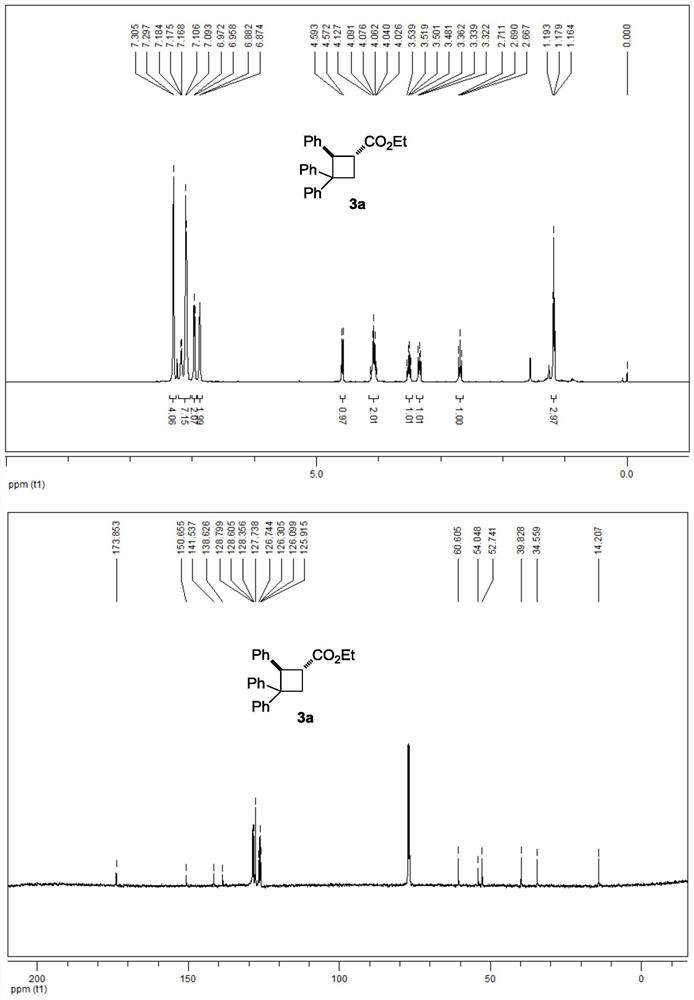
[0034] 3a NMR data:
[0035] 1 H NMR (500MHz, CDCl 3 ):δ7.30(d,J=4.0Hz,4H),7.18-7.09(m,7H),6.96(d,J=7.0Hz,2H),6.88(d,J=4.0Hz,2H),4.58 (d, J=10.5Hz, 1H), 4.13-4.03 (m, 2H), 3.51 (q, J=10.0Hz, 1H), 3.36-3.32 (m, 1H), 2.69 (t, J=11.0Hz, 1H), 1.18(t, J=7.0Hz, 3H)ppm.
[0036] 13 C NMR (125MHz, CDCl 3 ):δ173.9,150.7,141.5,138.6,128.8,128.6,128.4,127.7,126.7,126.3,126.1,125.9,60.6,54.0,52.7,39.8,34.6,14.2ppm.
Embodiment 2
[0038] The preparation of cyclobutane compound 3b, the reaction equation is as follows:
[0039]
[0040] Add compound 1a (100 mmol), compound 2b (500 mmol), Ir(ppy) to the reaction flask 3 (1mmol), Rh 2 (OOc) 4 (5 mmol), 1,2-dichloroethane (DCE, 500 mL), irradiated under a blue LED light for 12 h. After the reaction, the reaction solvent was removed using a rotary evaporator to obtain a crude product. The crude product was separated by column chromatography to obtain the target product 3b with a yield of 60% (3:1 d.r.).
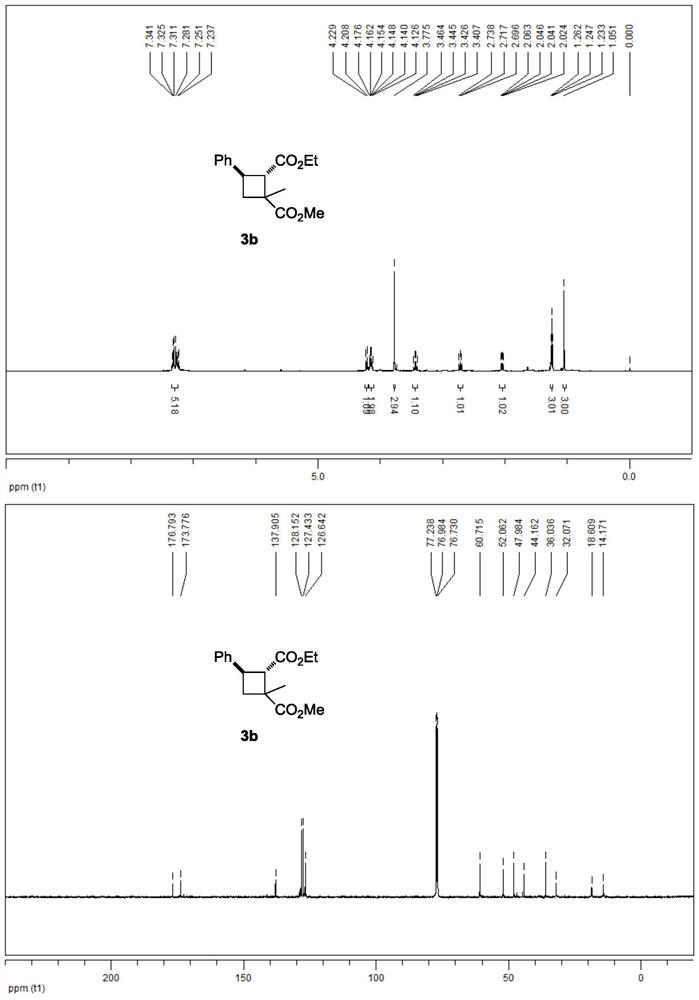
[0041] The 3b NMR data are as follows:
[0042] 1 H NMR (500MHz, CDCl 3 ): δ7.34-7.23(m, 5H), 4.22(d, J=10.5Hz, 1H), 4.18-4.13(m, 2H), 3.78(s, 3H), 3.44(q, J=9.5Hz, 1H), 2.72(t, J=10.5Hz, 1H), 2.04(dd, J=8.5, 11.0Hz, 1H), 1.25(t, J=7.5Hz, 3H), 1.05(s, 3H)ppm.
[0043] 13 C NMR (125MHz, CDCl 3 ): δ176.8,173.8,137.9,128.2,127.4,126.6,60.7,52.1,48.0,44.2,36.0,32.1,18.6,14.2ppm.
Embodiment 3
[0045] The preparation of cyclobutane compound 3c, the reaction equation is as follows:
[0046]
[0047] Add compound 1a (100 mmol), compound 2c (500 mmol), Ir(ppy) to the reaction flask 3 (1mmol), Rh 2 (OAc) 4 (5 mmol), 1,2-dichloroethane (DCE, 500 mL), irradiated under a blue LED light for 12 h. After the reaction, use a rotary evaporator to remove the reaction solvent to obtain a crude product. The crude product is separated by column chromatography to obtain the target product 3c with a yield of 85% (10:1 d.r.).
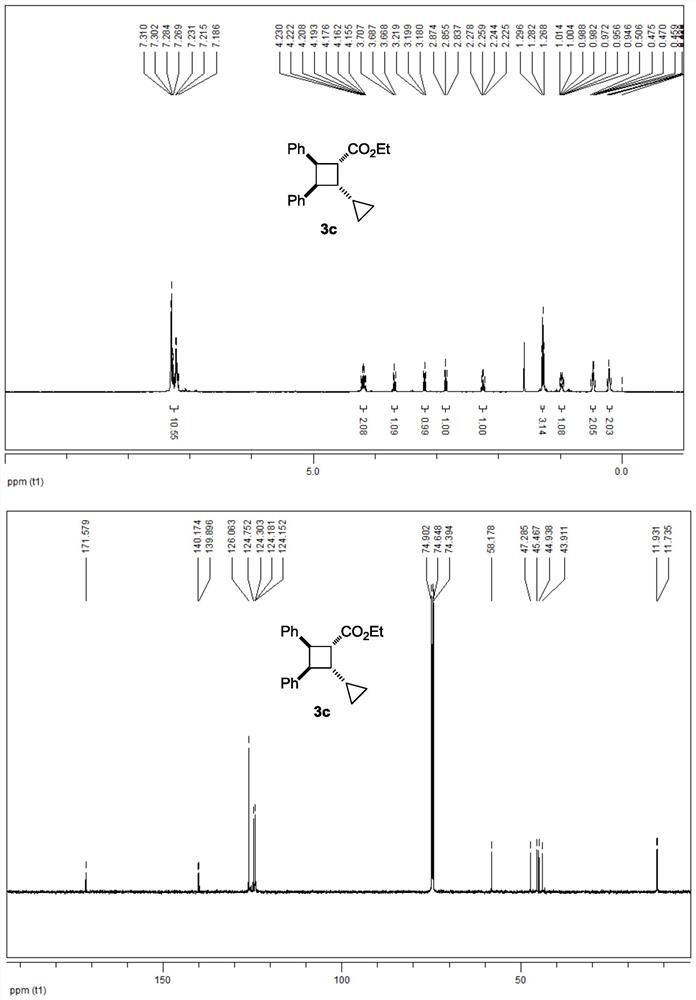
[0048] The 3c NMR data are as follows:
[0049] 1 H NMR (500MHz, CDCl 3 ): δ7.31-7.19(m, 10H), 4.23-4.16(m, 2H), 3.69(t, J=10.0Hz, 1H), 3.20(t, J=10.0Hz, 1H), 2.86(t, J=9.5Hz, 1H), 2.25(q, J=9.5Hz, 1H), 1.28(t, J=7.0Hz, 3H), 1.01-0.95(m, 1H), 0.51-0.43(m, 2H), 0.24-0.18(m,2H)ppm.
[0050] 13 C NMR (125MHz, CDCl 3 ): δ171.6,140.2,139.9,126.1,124.8,124.3,124.2,124.2,58.2,47.3,45.5,44.9,43.9,11.9,11.7ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com