Application of lncRNA SNHG7 in preparation of medicine for treating retinopathy
A technology for retinopathy and retina, applied in the field of application in the preparation of drugs for the treatment of retinopathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Materials and methods
[0037] 1.1 Cell culture and treatment
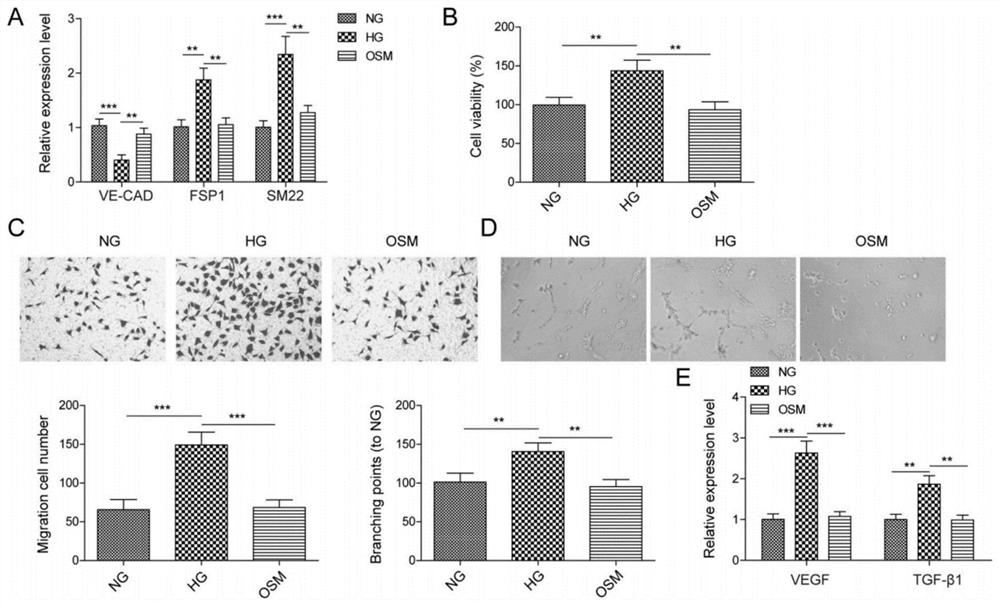
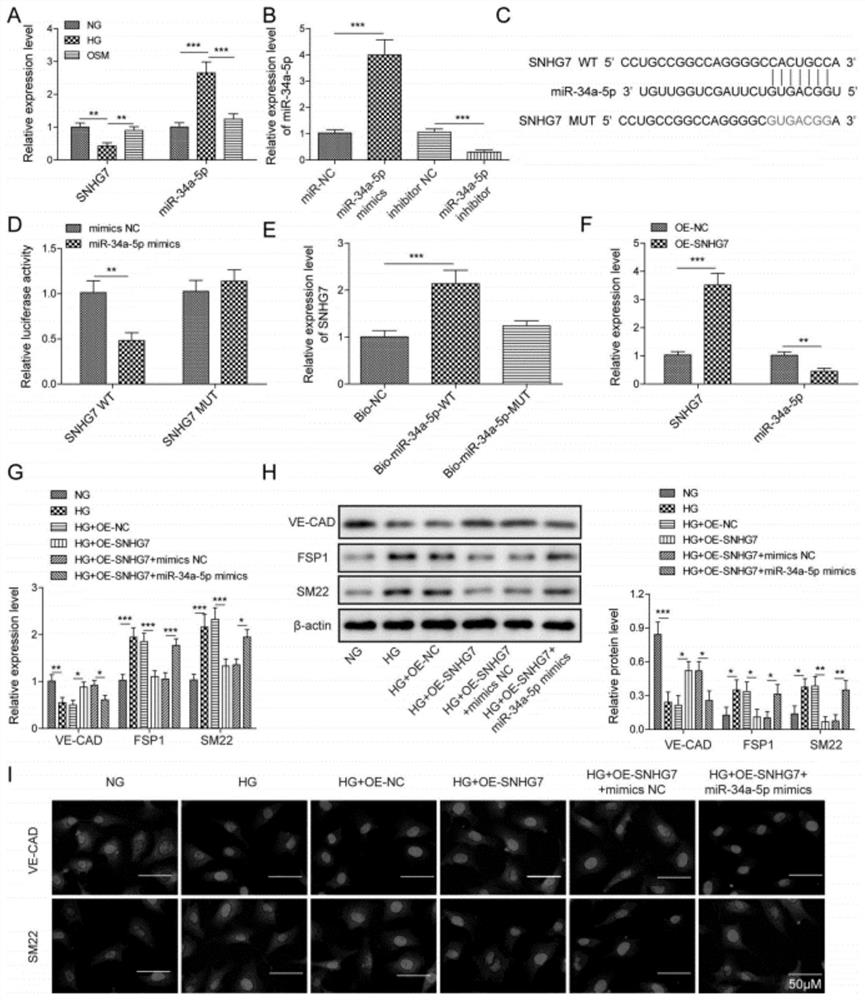
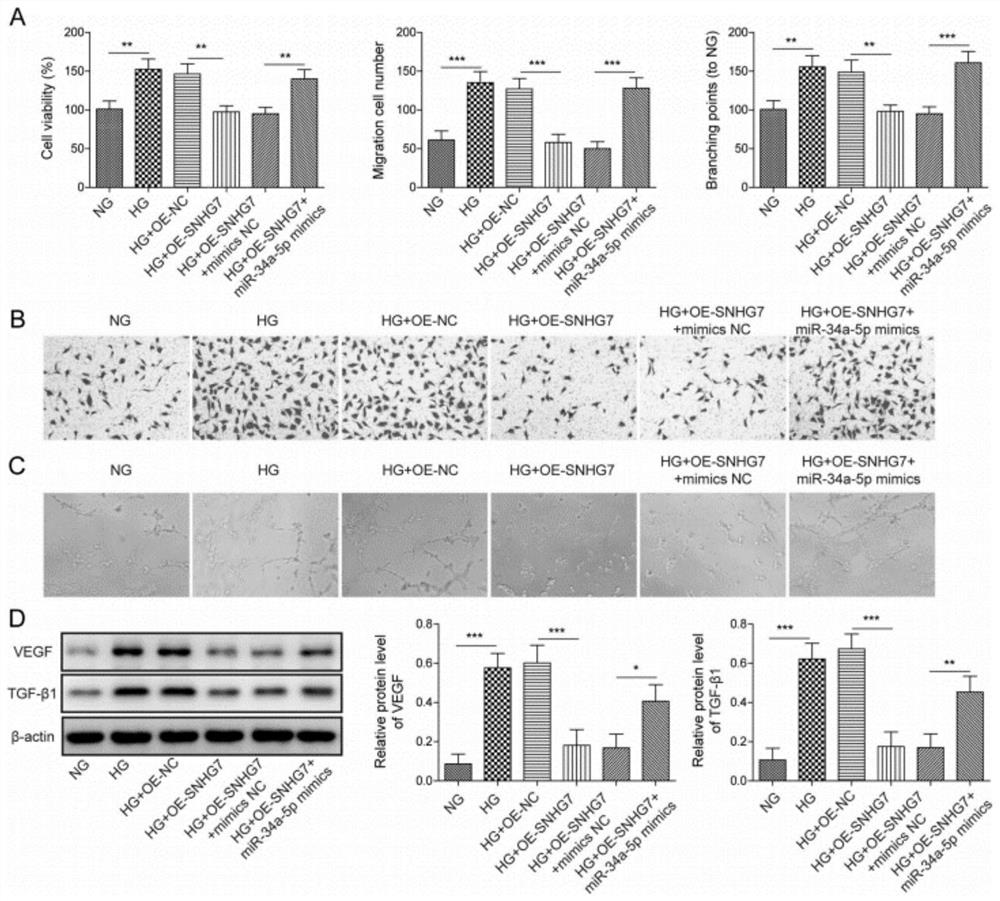
[0038] Human retinal microvascular endothelial cells (HRMECs) were obtained from the American Type Culture Collection (USA) and grown in the presence of 10% fetal bovine serum (FBS; Invitrogen, USA), 1% penicillin / streptomycin (Invitrogen), endothelial cells Factor (EGM; SingleQuots, Clonetics, San Diego, USA) in endothelial basal medium (LonzaGroup, Switzerland); cells were incubated in 5% CO 2 Maintain the culture at 37 °C under humidified conditions. For high glucose treatment, HRMECs treated with 30 mM dextrose ((Sigma, USA) for 48 h, treated with normal glucose (5.5 mM) or mannitol (24.5 mM) plus glucose (5.5 mM) were used as controls. For cells For transfection, OE-NC (negative control plasmid), OE-SNHG7, miR-NC, miR-34a-5p mimic, inhibitor NC, miR-34a were transfected with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) -5p inhibitor, sh-NC, sh-XBP1 delivered in the cell.24 hours after the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com