AMYLOID PRECURSOR PROTEIN (APP) RNAi AGENT COMPOSITIONS AND METHODS OF USE THEREOF
A precursor protein, amyloid technology, applied in DNA/RNA fragments, biochemical equipment and methods, single-stranded DNA viruses, etc., can solve problems such as limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0350] Additional representative U.S. patents and U.S. patent publications that teach the preparation of locked nucleic acid nucleotides include, but are not limited to, the following: U.S. Patent No. 6,268,490; U.S. Patent No. 6,525,191; U.S. Patent No. 6,670,461; U.S. Patent No. 6,770,748; U.S. Patent No. 6,794,499; U.S. Patent No. 6,998,484; U.S. Patent No. 7,053,207; U.S. Patent No. 7,034,133; U.S. Patent No. 8,022,193; U.S. Patent No. 8,030,467; U.S. Patent No. 8,278,425; U.S. Patent No. 8,278,426; U.S. Patent No. 8,278,283; The entire content of one article is incorporated herein by reference.
[0351] Any of the foregoing bicyclic nucleosides may be prepared with one or more stereochemical sugar configurations, including for example alpha-L-ribofuranose and beta-D-ribofuranose (see WO 99 / 14226).
[0352] The modified RNAi agents disclosed herein can also include one or more restricted ethyl nucleotides. As used herein, a "constrained ethyl nucleotide" or "cEt" is a lo...
example
[0918] Example 1. RNAi reagent design, synthesis, selection and in vitro evaluation
[0919] This example describes methods for the design, synthesis, selection and in vitro evaluation of APP RNAi agents.
[0920] Reagent source
[0921] Where the source of an agent is not specifically given herein, such agents can be obtained from any supplier of molecular biology agents in quality / purity standards for molecular biology applications.
[0922] Bioinformatics
[0923] siRNA agents targeting a group of human amyloid-beta precursor genes (APP; human NCBI refseqNM_201414; NCBI GeneID:351; SEQ ID NO:1), and APP orthologues in the toxicology species from cynomolgus monkeys ( Cynomolgus monkey: XM_005548883.2; SEQ ID NO:12) was designed using custom R and Python. All siRNA designs were perfectly matched to the human APP transcript, and a subset were perfectly or near-perfectly matched to the cynomolgus monkey ortholog. Human NM_201414 REFSEQ mRNA, version 2, 3423 bases in length....
example 2
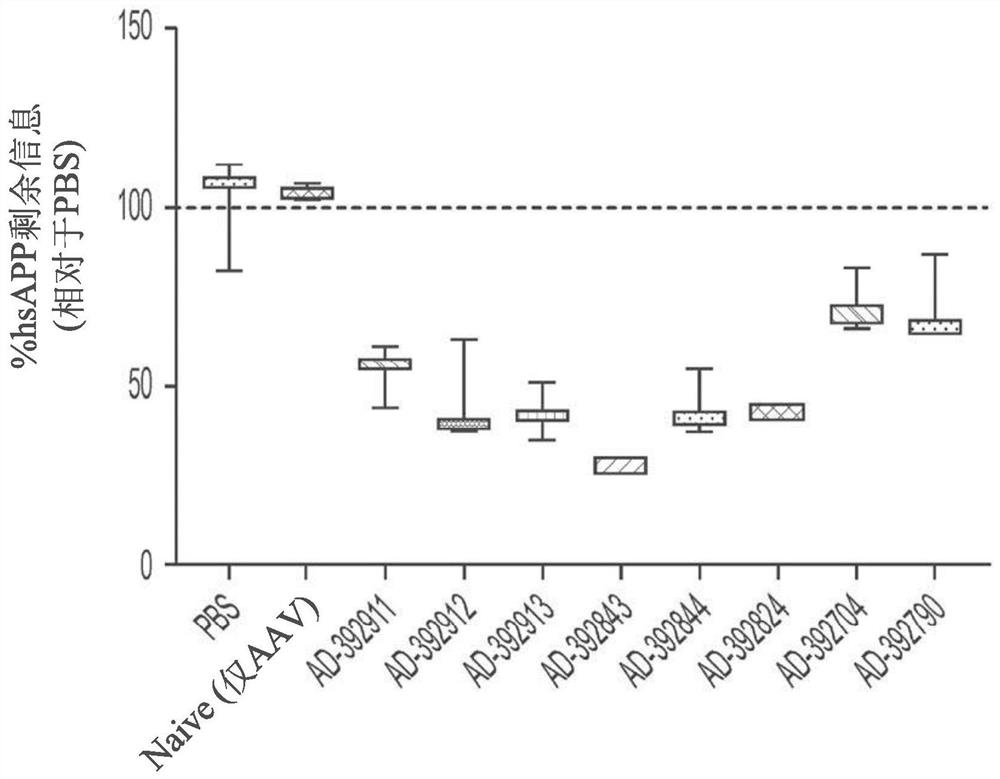
[1016] Example 2: In vivo evaluation of RNAi agents
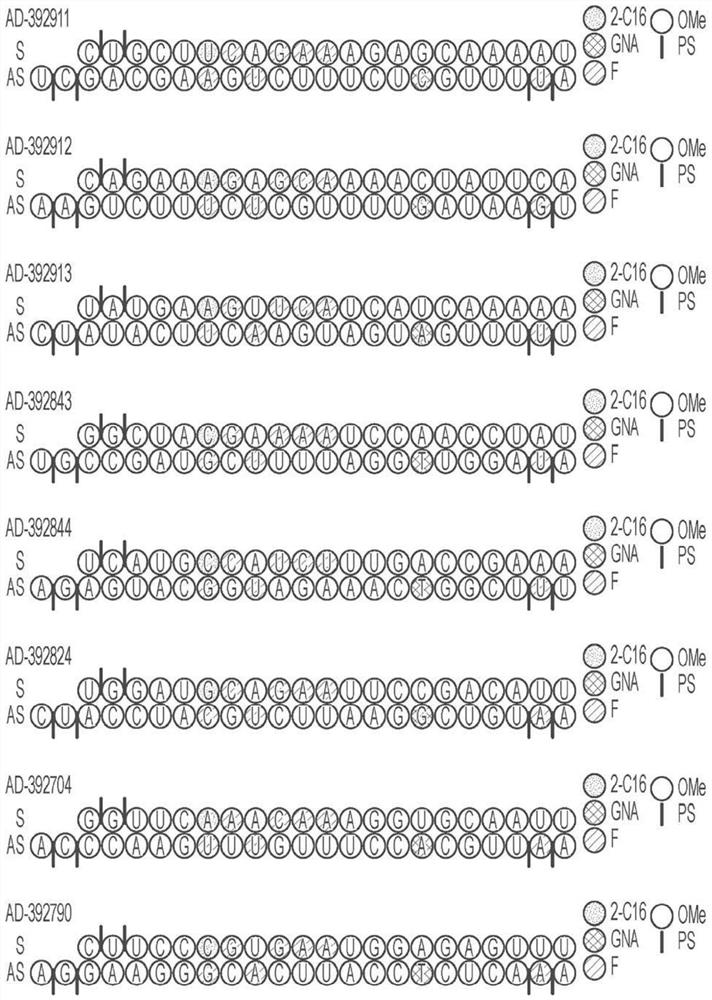
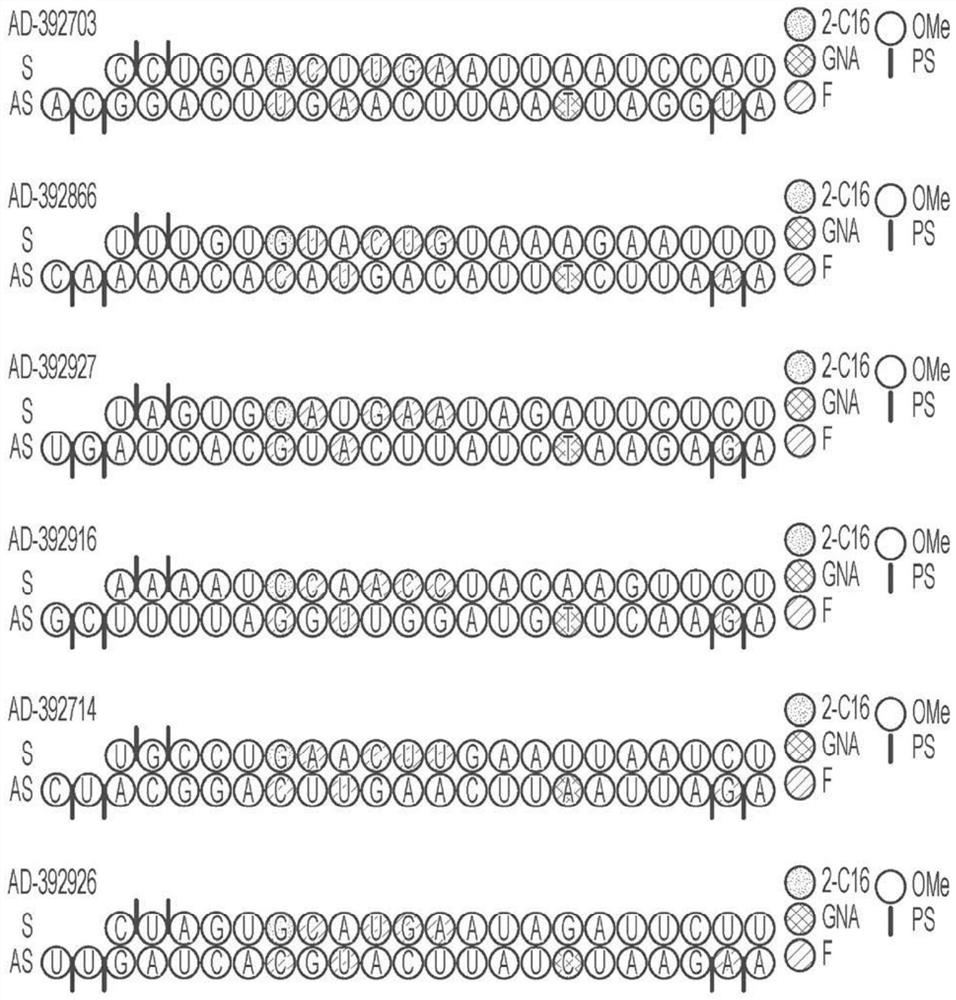
[1017] Selected APP-targeting RNAi agents were evaluated for in vivo efficacy in a proof-of-concept and lead identification screen for human APP knockout in AAV mice. Selected RNAi agents for such studies include AD-392911, AD-392912, AD-392911, AD-392912, AD-392913, AD-392843, AD-392844, AD-392824, AD-392704, AD-392790, AD-392703, AD-392866, AD-392927, AD-392916, AD-392714 and AD-392926, having the sequence as above in Table 2A of the right, the corresponding unmodified sequence as in Table 3 above, and As depicted graphically in Fig. 1A and Fig. In Figure 1B, each of the RNAi agents tested in this example further presents a triantennary GalNAc moiety attached at the 3' residue of the sense strand in order to aid the liver targeting of such RNAi agents when subcutaneously administered to mice (for intrathecal administration, the lack of a conjugated GalNAc moiety is also explicitly considered).
[1018] In such studies...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com