Product based on intracellular mycobacterium pure protein derivative and recombinant mycobacterium tuberculosis fusion protein, application and use method
A technology of protein derivatives and mycobacteria, applied in the field of biological proteins, can solve the problems of affecting the treatment of patients, different treatment plans, time-consuming, etc., and achieve reliable theoretical basis and reference basis, short identification time and high significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
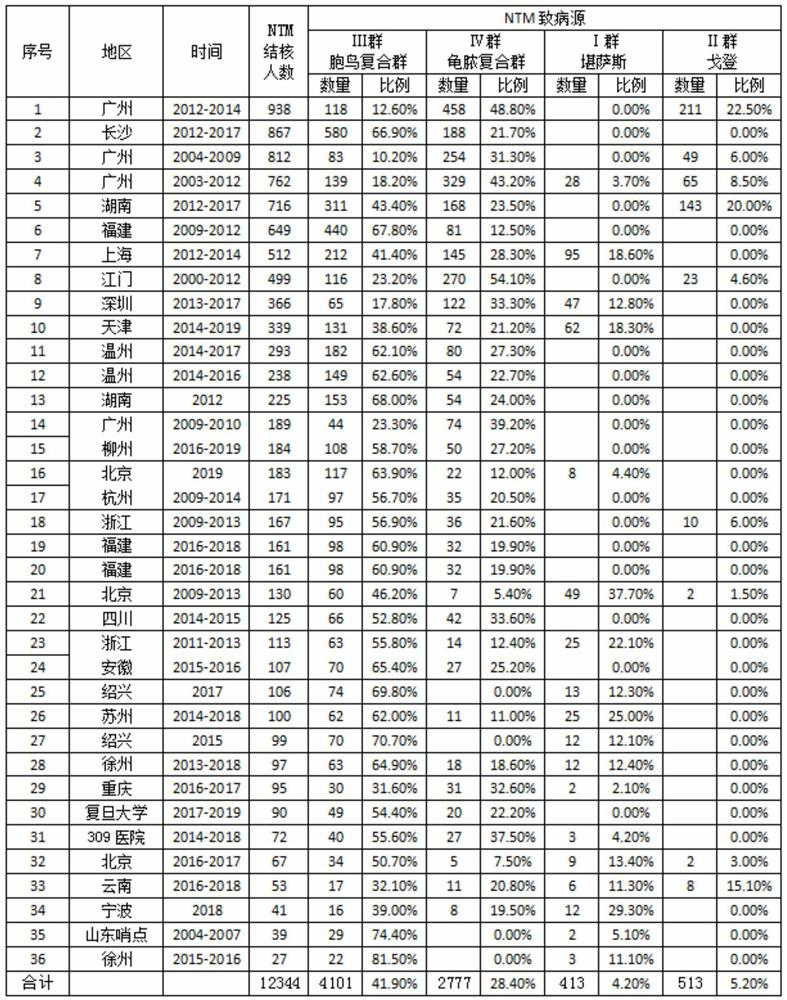
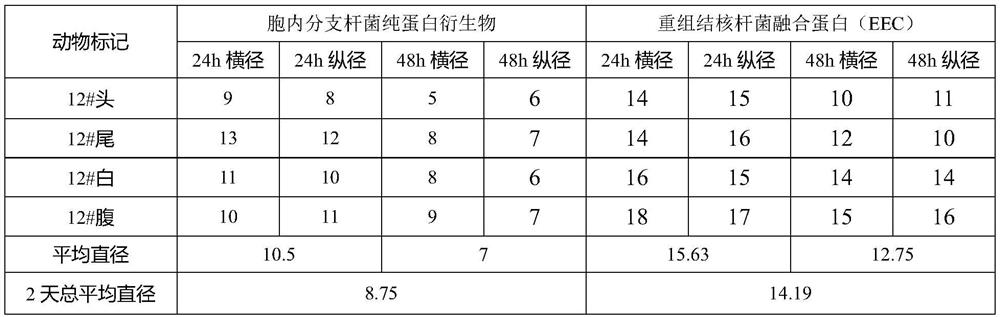
[0040] This example discusses the combination of pure protein derivatives of Mycobacterium intracellulare and recombinant Mycobacterium tuberculosis fusion protein to induce delayed hypersensitivity in guinea pigs sensitized to the attenuated strain of Mycobacterium tuberculosis (CMCC93020), so as to further improve the technical scheme illustrate.
[0041] S1: guinea pig
[0042] SPF grade guinea pigs with a body weight of 300-500g were raised in IVC cages (3 guinea pigs per cage, the litter was changed twice a week, free access to food and water).
[0043] S2: skin test
[0044] Tuberculin pure protein derivative skin test negative guinea pigs.
[0045] S3: Allergens
[0046] Take the sensitizer (50mg / mL) of the attenuated strain of Mycobacterium tuberculosis (CMCC93020), and sterilize it at 121°C for 20min.
[0047] S4: Sensitization
[0048] Inject 0.2mL of the allergenic bacteria solution (50mg / mL) in the groin, and perform the second sensitization 3 weeks after the ...
Embodiment 2
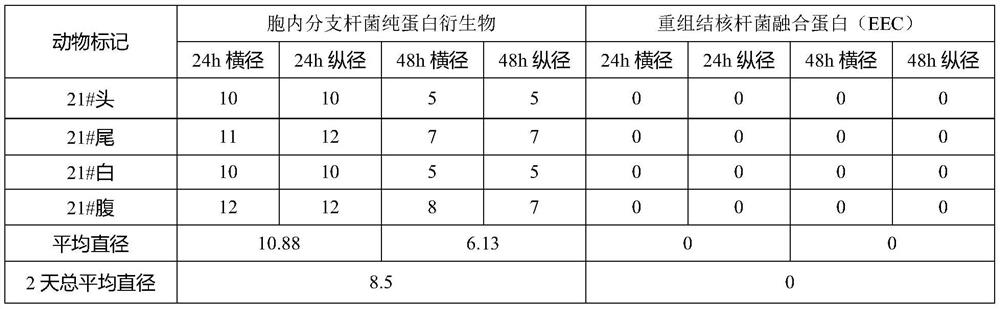
[0055] This example discusses the combination of pure protein derivatives of Mycobacterium intracellulare and recombinant Mycobacterium tuberculosis fusion protein against Mycobacterium intracellulare (CMCC 95002) and Guinea pigs sensitized with Mycobacterium avium (CMCC95001) induced delayed hypersensitivity to further illustrate the technical solution.
[0056] S1: guinea pig
[0057] SPF grade guinea pigs with a body weight of 300-500g were raised in IVC cages (3 guinea pigs per cage, the litter was changed twice a week, free access to food and water).
[0058] S2: skin test
[0059] Tuberculin pure protein derivative skin test negative guinea pigs.
[0060] S3: Allergens
[0061] Allergens (50 mg / mL) of Mycobacterium intracellulare (CMCC 95002) and Mycobacterium avium (CMCC95001) were taken and sterilized at 121°C for 20 minutes.
[0062] S4: Sensitization
[0063] Inject 0.2mL of the allergenic bacteria solution (50mg / mL) in the groin, and perform the second sensitiz...
Embodiment 3
[0072] This example discusses the combination of pure protein derivatives of Mycobacterium intracellulare and recombinant Mycobacterium tuberculosis fusion protein against Mycobacterium kansasii (CMCC 95013) and Hai Mycobacterium (CMCC 95014) and simian mycobacterium (CMCC95015) sensitized guinea pigs induced delayed hypersensitivity reactions to further illustrate this technical solution.
[0073] S1: guinea pig
[0074] SPF grade guinea pigs with a body weight of 300-500g were raised in IVC cages (3 guinea pigs per cage, the litter was changed twice a week, free access to food and water).
[0075] S2: skin test
[0076] Tuberculin pure protein derivative skin test negative guinea pigs.
[0077] S3: Allergens
[0078] Allergens (50 mg / mL) of Mycobacterium kansasii (CMCC 95013), Mycobacterium marinum (CMCC 95014) and Mycobacterium simianus (CMCC95015) were taken and sterilized at 121°C for 20 minutes.
[0079] S4: Sensitization
[0080] Inject 0.2mL of the allergenic bact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com