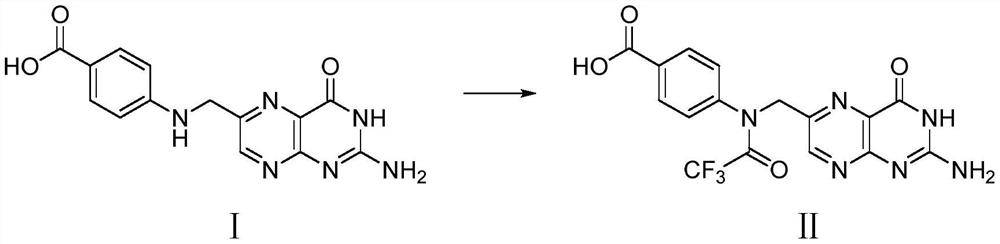
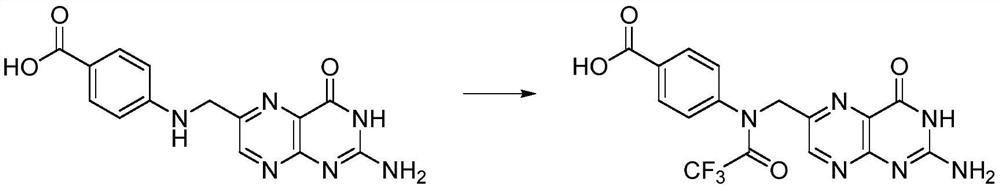
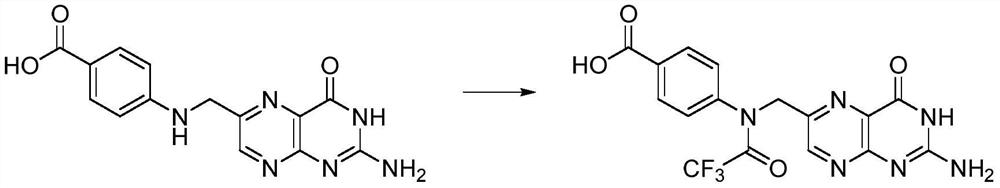
Preparation method of N10-trifluoroacetyl pteroic acid
A technology of trifluoroacetyl pteroic acid and N10-, which is applied in the field of medicine and chemical industry, can solve the problems of high toxicity of trifluoroacetic anhydride, unfavorable large-scale production, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] In a 500mL three-necked flask under the protection of inert gas, add 19.5g (50mmmol) instant pteroic acid (80% content), 200mL DMSO, stir well, then add 12.6g (60mmmol) trifluoroacetic anhydride, 20-25°C Stir in the dark for 12 hours, add the filtrate to a mixture of 300 mL ethyl acetate and 300 mL deionized water, stir evenly, and separate phases. Extract the organic phase with 100mL ethyl acetate once more, combine the organic phases, dry over anhydrous magnesium sulfate, filter, evaporate the filtrate to about 100mL under vacuum, stir to remove crystals, cool to 5°C, filter, rinse with 100mL cold ethyl acetate filter cake. The filter cake was vacuum-dried (protected from light) to remove the organic solvent to obtain a yellow solid as compound II, N 10 - Trifluoroacetyl pteroic acid 16.5g, yield 81%.
Embodiment 2
[0026]
[0027] In the 500mL three-neck flask under the protection of inert gas, add 9.75g (25mmmol) instant pteroic acid (content is 80%), add 7.8g (25mmmol) anhydrous pteroic acid, 200mL DMSO, stir evenly, then add 12.6g ( 60mmmol) trifluoroacetic anhydride, and stirred in the dark at 20-25°C for 12 hours, and the filtrate was added to a mixture of 300mL ethyl acetate and 300mL deionized water, stirred evenly, and phase-separated. Extract the organic phase with 100mL ethyl acetate once more, combine the organic phases, dry over anhydrous magnesium sulfate, filter, evaporate the filtrate to about 100mL under vacuum, stir to remove crystals, cool to 5°C, filter, rinse with 100mL cold ethyl acetate filter cake. The filter cake was vacuum-dried (protected from light) to remove the organic solvent to obtain a yellow solid as compound II, N 10 - Trifluoroacetyl pteroic acid 15.3g, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com