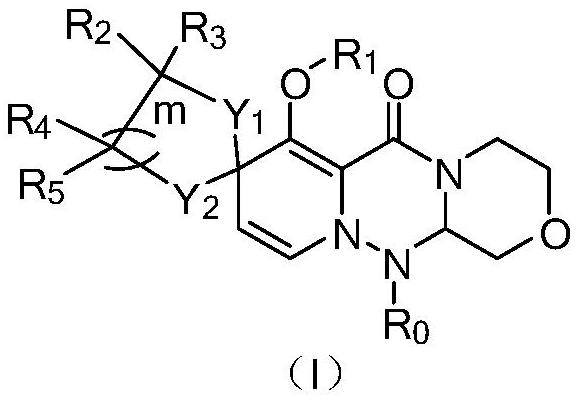
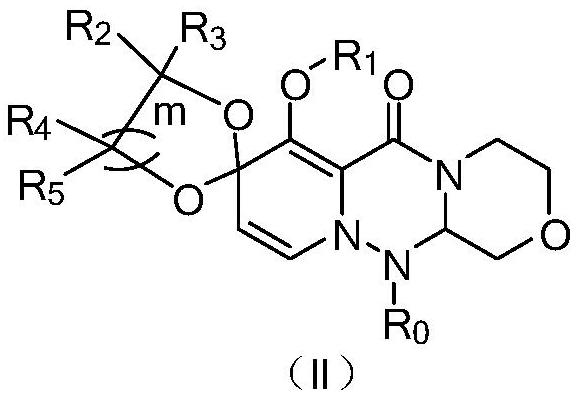
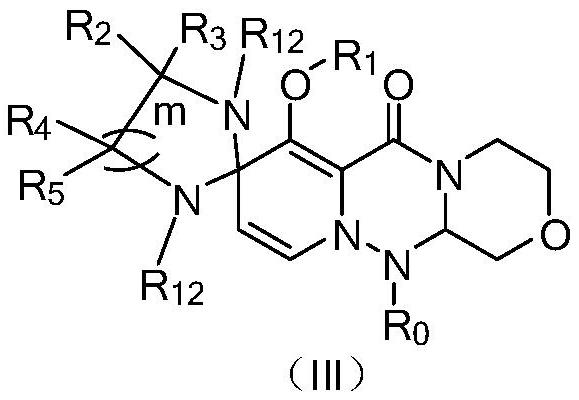
Polycyclic compound and pharmaceutical composition and application thereof
A technology for polycyclic compounds and compounds, used in organic chemistry, pharmaceutical formulations, bulk chemical production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087]
[0088] Synthesis of compound 1b:
[0089] Add 5g of compound 1a, 65mg of p-toluenesulfonic acid, 2.7g of ethylene glycol, and 50ml of benzene into the reaction flask, and heat the system to reflux with a water separator for 24 hours. The system was concentrated to dryness, dichloromethane and water were added, the organic phase was separated, concentrated to dryness and separated by a silica gel column to obtain 3.94g of compound 1b with a yield of 72%, ESI-MS (+): m / z 506.19 [M +H];
[0090] Synthesis of compound 1c:
[0091] Add 3.8g of compound 1b, 30ml of methanol and 0.45g of 10% palladium carbon into the reaction flask, and hydrogenate at room temperature and pressure for 24 hours. After the reaction, the system was filtered with diatomaceous earth; the system was concentrated to dryness under reduced pressure, and purified by silica gel column to obtain 1.9 g of compound 1c, with a yield of 90%, ESI-MS (+): m / z 282.12 [M+H];
Embodiment 2
[0093]
[0094] Synthesis of compound 2b:
[0095]Add 5g of compound 1a, 65mg of p-toluenesulfonic acid, 4.5g of 2-hydroxy-2-methylpropionic acid, and 50ml of benzene into the reaction flask, and heat the system to reflux for 24 hours with a water separator. The system was concentrated to dryness, dichloromethane and water were added, the organic phase was separated, concentrated to dryness and separated by a silica gel column to obtain 4.03g of compound 2b with a yield of 68%, ESI-MS (+): m / z548.22[ M+H];
[0096] Synthesis of compound 2c:
[0097] Add 3.8g of compound 2b, 30ml of methanol and 0.41g of 10% palladium carbon into the reaction flask, and hydrogenate at room temperature and pressure for 24 hours. After the reaction, the system was filtered with diatomaceous earth; the system was concentrated to dryness under reduced pressure, and purified by silica gel column to obtain 2.04g of compound 2c, with a yield of 91%, ESI-MS (+): m / z 324.10 [M+H];
Embodiment 3
[0099]
[0100] Synthesis of compound 3b:
[0101] Add 5g of compound 1a, 65mg of p-toluenesulfonic acid, 3.86g of 2-amino-2-methylpropanol, and 50ml of benzene into the reaction flask, and heat the system to reflux for 24 hours with a water separator. The system was concentrated to dryness, dichloromethane and water were added, the organic phase was separated, concentrated to dryness and separated by a silica gel column to obtain 4.33g of compound 3b, yield 75%, ESI-MS (+): m / z 533.21 [M +H];
[0102] Synthesis of compound 3c:
[0103] Add 3.8g of compound 3b, 30ml of methanol and 0.42g of 10% palladium carbon into the reaction bottle, and hydrogenate at room temperature and pressure for 24 hours. After the reaction, the system was filtered with diatomaceous earth; the system was concentrated to dryness under reduced pressure, and purified by silica gel column to obtain 1.94g of compound 3c, with a yield of 88%, ESI-MS (+): m / z 309.13 [M+H];
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com