ZjAC2 coding gene sequence with adenosine triphosphate tunneling metalloenzyme activity
An adenosine triphosphate tunneling and gene-encoding technology, which is applied in the field of adenosine triphosphate tunneling metalloenzymes, and can solve problems such as insufficient understanding of TTM proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Amplification of the ZjAC2 gene
[0021] The RNA of winter jujube fruit was extracted, and then reverse transcribed into cDNA as a PCR amplification template for PCR amplification; among them, the forward primer: 5'-ATGGAGGTCGAAGTCAAGCT-3', and the reverse primer: 5'-CTAAGGCAGCTTTCCGGATC-3'.
[0022] PCR amplification program: 94°C, 4 min; (94°C, 40s; 56°C, 45s; 72°C, 50s) 35 cycles; 72°C, 10 min.
[0023] The PCR products were subjected to agarose gel electrophoresis to observe the amplification results, and the candidate target bands were recovered by agarose gel. The recovered products were ligated into the pMDTM19-T cloning vector for sequencing, and the ZjAC2 sequence was obtained as a candidate for jujube adenylate cyclization. Enzyme gene for functional verification.
Embodiment 2
[0025] Escherichia coli Deficient Complementation Assay
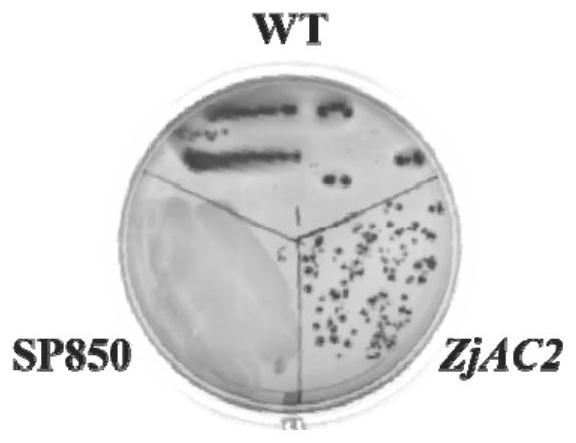
[0026] E. coli cyaA-deficient strain SP850 was cultured. The SP850 strain lacked the adenylate cyclase (AC) gene. The pET-15b recombinant plasmid with the ZjAC2 gene was heat-shocked into SP850, and then coated with ampicillin and kana antibiotics. culture dish, pick single clones and culture to OD600 of 0.5, then induce expression and culture for 4h, streak culture in MacConkey medium and observe.
[0027] It was found that if figure 1 , the colony color of SP850-deficient strain and wild-type E. coli strain with recombinant plasmid are red, while the color of SP850-deficient strain in MacConkey medium is white, indicating that ZjAC2 gene makes up for the lack of cyaA in SP850 strain gene that functions as adenylate cyclase gene.
Embodiment 3
[0029] Protein in vitro catalytic assay
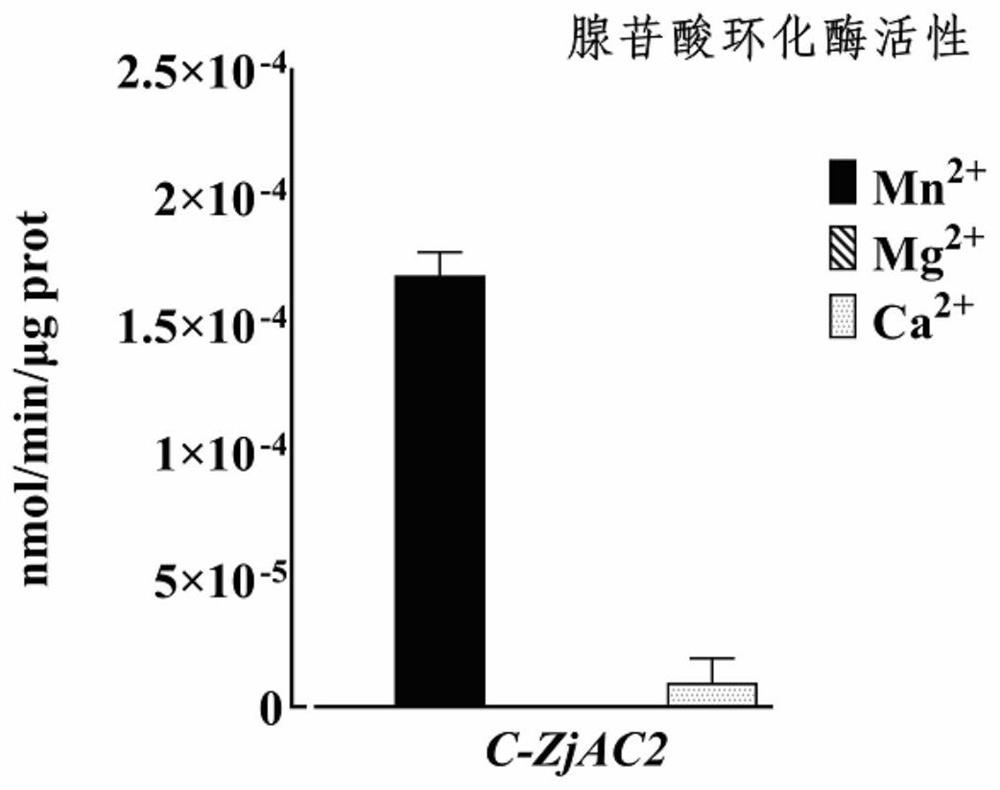
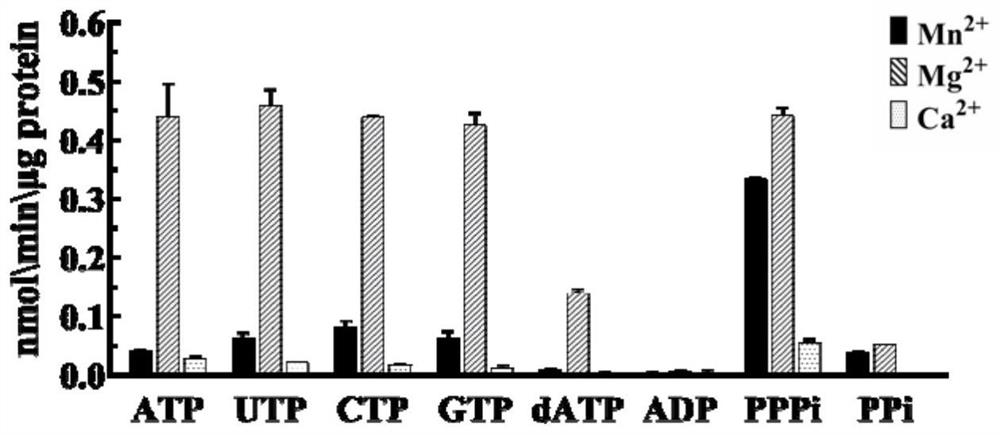
[0030] The pET-15b recombinant vector with ZjAC2 gene was transferred into Escherichia coli BL21 strain, cultured at 37°C, 200rpm, after induction of protein expression by IPTG, protein extraction and purification was performed, and the obtained protein was used for enzyme catalytic activity detection. The results are as follows Figure 2-4 . It was found that Mn 2+ and Ca 2+ When used as a coenzyme ion, cAMP was generated in the catalytic system, indicating that ZjAC2 protein can catalyze ATP to form cAMP and play the function of AC; in addition, in Mg 2+ When used as coenzyme ion, there is still a lot of Pi in the reaction product, but after adding pyrophosphohydrolase, the Pi content does not increase, indicating that ZjAC2 protein hydrolyzes ATP into ADP and PPi, while Mn 2+ and Ca 2+ When used as a coenzyme ion, the content of Pi in the reaction product was relatively small, but after the addition of pyrophosphatase, the conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com