Sterility detection method for pipeline and container medical instruments
A technology for medical devices and detection methods, applied in the medical and health field, can solve the problems of false positives, false negatives, complicated operations, etc., and achieve the effects of high detection rate, improved detection rate, and simple operation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
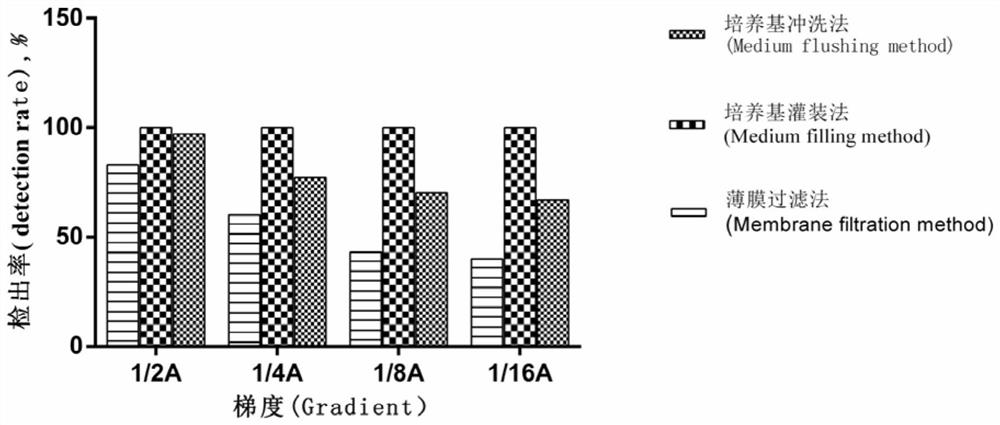
[0031] The sterility testing method for tubing-type medical devices uses the culture medium flushing method to perform sterility testing on infusion sets, including the following steps:
[0032] (1) Insert the puncture needle of the infusion set (model specification: A 0.55×19RW LB) into the mouth of the bottle containing trypticase soytone liquid medium or thioglycolate fluid medium, so that the medium flows through the inner cavity of the tube , the flow rate is about 10mL / min, collect 100mL of washing liquid in a sterile culture bottle.
[0033] In order to facilitate the calculation of the detection rate, the infusion set in this step has been artificially polluted in advance, and the specific operation is as follows:
[0034] a. Preparation of test bacteria suspension
[0035] Use a pipette gun to take 0.1mL with a concentration of 1.9×10 8 / 0.1mL of ATCC9372 bacteria suspension was added to 9.9mL 40% alcohol solution to make 1.9×10 6 / 0.1mL of bacterial suspension, an...
Embodiment 2
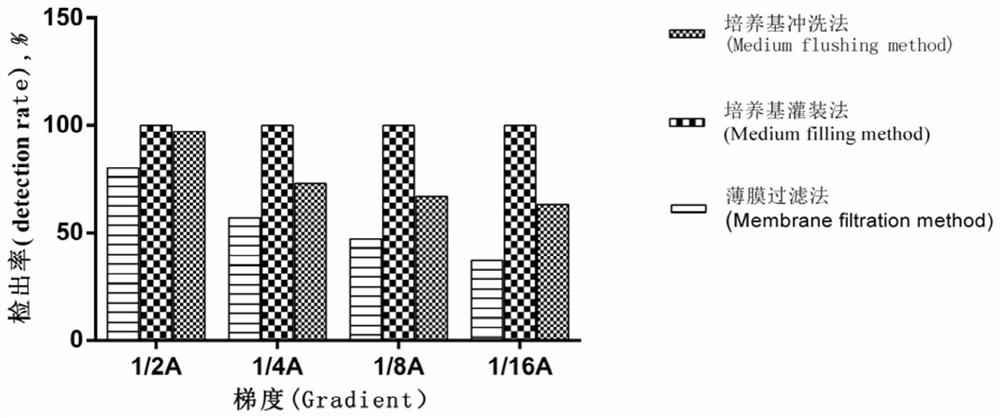
[0046] The sterility test method for container medical devices uses the culture medium flushing method to test the sterility of blood bags, including the following steps:
[0047] (1) Use a disposable sterile syringe to add 100 mL of TSB medium or FTM medium into a disposable plastic blood bag, and then shake it manually for 2 minutes;
[0048] In order to facilitate the calculation of the detection rate, the blood bag in this step has been artificially polluted in advance, and the specific operation is as follows:
[0049] a. Preparation of test bacteria suspension
[0050] Use a pipette gun to take 0.1mL with a concentration of 1.9×10 8 / 0.1mL of ATCC9372 bacteria suspension was added to 9.9mL 40% alcohol solution to make 1.9×10 6 / 0.1mL of bacterial suspension, and serially diluted to 1.9×10 4 / 0.1mL, 1.9×10 2 / 0.1mL, the concentration will be 1.9×10 2 / 0.1mL of bacterial suspension is recorded as A (gradient 1'), take 5mL of bacterial suspension A and add it to 5mL of 4...
Embodiment 3
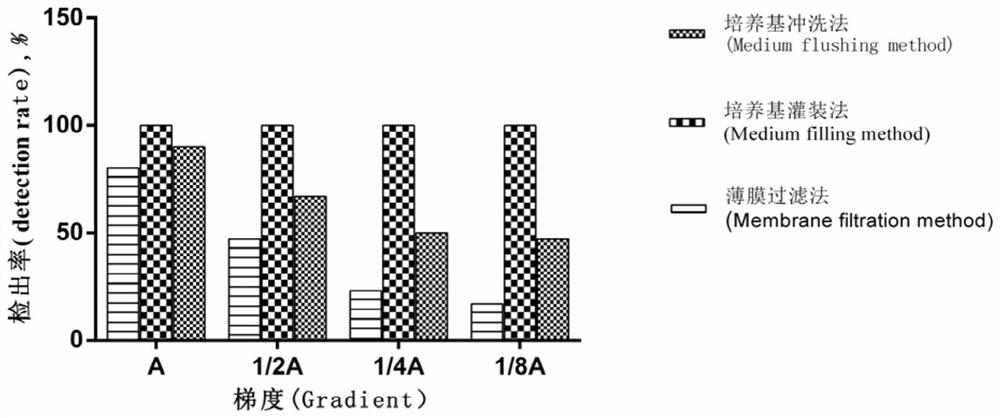
[0061] The sterility testing method for tubing-type medical devices uses the culture medium filling method to perform sterility testing on infusion sets, including the following steps:
[0062] (1) Pierce the puncture needle of the infusion set with the same model as in Example 1 into the mouth of the bottled TSB medium or FTM medium bottle, make the flushing liquid flow into and fill the inner cavity of the infusion tube, cover the puncture needle end with a protective cap, and the other end Use clips to prevent media from flowing out.
[0063] In order to facilitate the calculation of the detection rate, the infusion set in this step has been artificially polluted in advance, and the specific operation is the same as that in Example 1.
[0064] (2) The infusion set containing the culture medium was placed in an incubator and cultured for 14 days, the culture temperature of the TSB medium was 22.5°C, and the culture temperature of the FTM medium was 32.5°C.
[0065] (3) Visu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com