Cancer-targeted, virus-encoded, regulatable t (catvert) or nk cell (catvern) linkers
A technology that encodes, immune effector cells in the field of cancer-targeted, virus-encoded, regulated T-cell (CATVERT) or NK-cell (CATVERN) linkers to address side effects, gene inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0497] Example 1 - Generation of CD3xGD2-HDD Dimert Using Exemplary CD3xGD2-HDD TransJoin
[0498] Map of exemplary AAV constructs used to test CD3xGD2-HDD expression.
[0499] As a proof of principle, a previously described bispecific molecule targeting human CD3 on T cells and the disialoganglioside GD2 on neuroblastoma and other types of cancer cells was expressed. This bispecific protein has the amino acid sequences of the heavy and light chain variable regions of human CD3 derived from clone OKT3 (using a single-chain variable fragment format scFv), fused via a short linker (L) to the scFv construct against GD2 of 5F11 linked as a dimer via the HNF1a dimerization domain (HDD) as in Ahmed et al ., OncoImmunology , 4:4, e989776, DOI: 10.4161 / 2162402X.2014.989776 as reported. The vectorbuilder.com codon optimization tool (http: / / en.vectorbuilder.com / tool / codon-optimization.html) was used to reverse engineer the optimal human DNA coding sequence for CD3xGD2-HDD. The res...
Embodiment 2
[0523] Example 2 - Generation of CD19xCD3 Dimert using an exemplary CD19xCD3 TransJoin
[0524] Map of exemplary AAV constructs used to test CD19xCD3 Dimert expression.
[0525] The FDA-approved protein therapeutic, called blinatumomab, is a so-called bispecific T-cell engager (BiTE) that targets human CD19 and Human CD3 on T cells. Using the publicly available amino acid sequence of blinatumomab (http: / / www.drugbank.ca / drugs / DB09052) and further using the vectorbuilder.com codon optimization tool (http: / / en.vectorbuilder.com / tool / codon-optimization. html) to reverse engineer the optimal human DNA coding sequence for CD19xCD3. The resulting DNA sequence starting with the ATG start codon was synthesized and cloned into an adeno-associated virus expression cassette downstream of the chicken-actin-b-globin promoter (CAGp) with inverted terminal repeats derived from AAV2 sequence. Based on the discovery that alanine enhances secretion of proteins (Güler-Gane et al ., PLoS ...
Embodiment 3
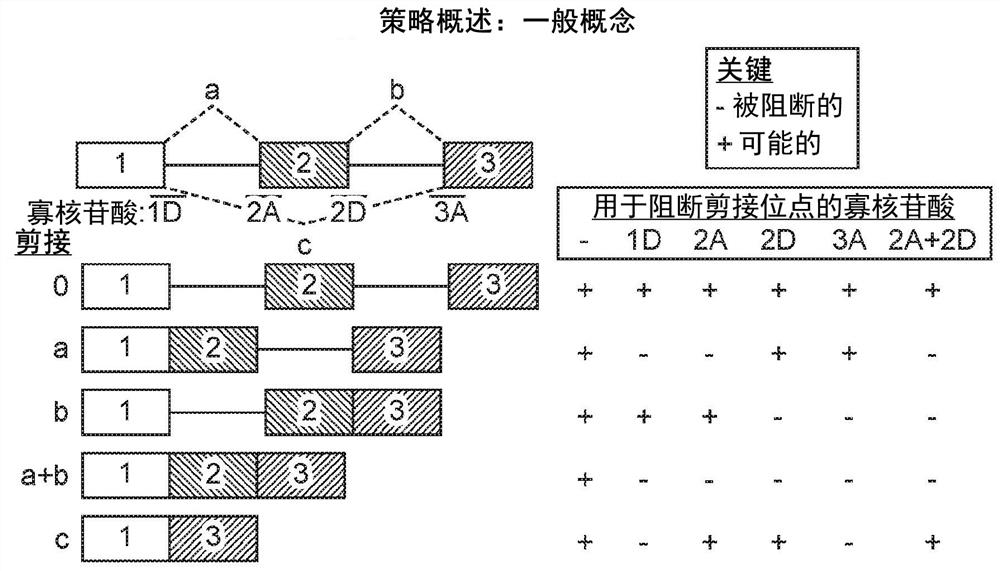
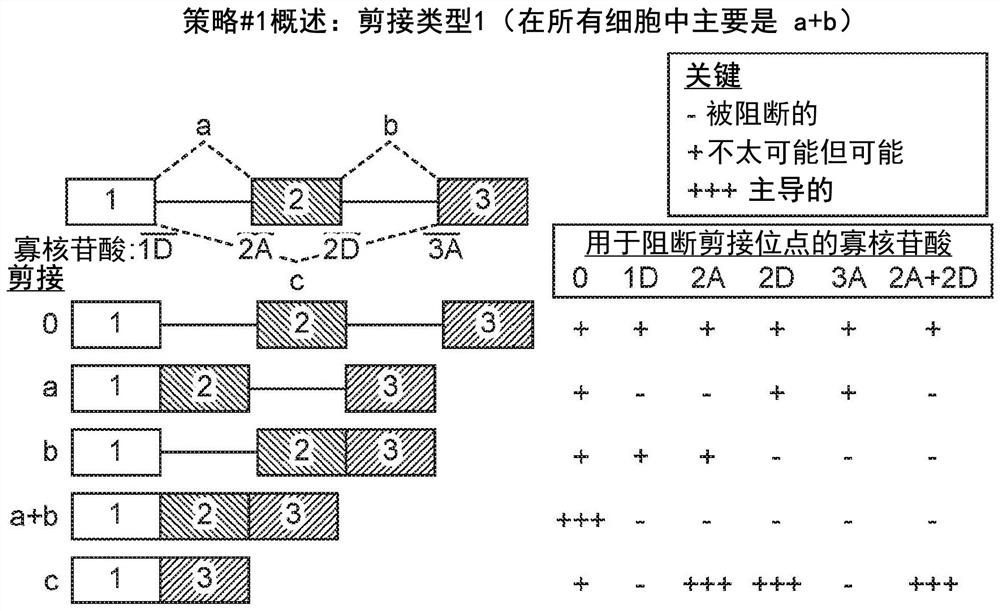
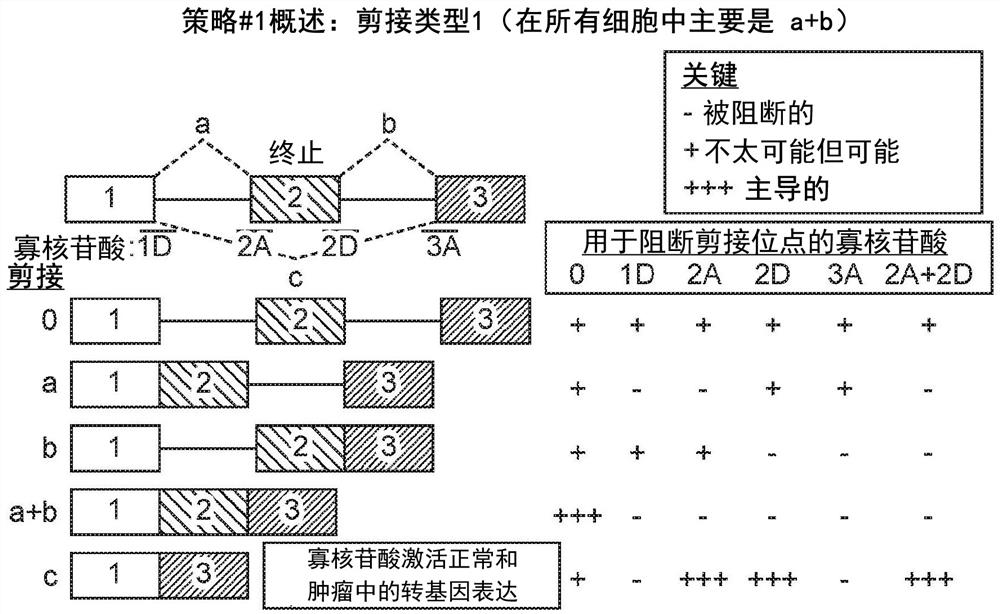
[0555] Example 3 - Using OncoSkip and TransSkip to Simultaneously Target Oncogene Expression and Activate Therapeutic Transgene Expression
[0556] An overview of OncoSkip and TransSkip described in this article.
[0557] OncoSkip is an antisense morpholino that induces exon skipping in oncogenes. In some embodiments, OncoSkip is designed as an antisense morpholino that induces in oncogenes ( Figure 30 Left side of ) Exon skipping of key exons. For a proof-of-principle, KRAS (exons 1, 2, 3, by Figure 30 black-gold-gray on the left) and tested the morpholino group designed to skip KRAS exon 2 ( Figure 30 , shown as light gray bars on top of oncogenes), which contain the ATG initiation site, so normal expression of the oncogene is reduced. Derivatives of the same exons to be skipped were then created, but mutated to include multiple stop codons in each reading frame as well as flanking intronic sequences. A new intron-exon (STOP)-intron is then inserted into the transgen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com