KCNH2 mutant gene and long QT syndrome detection kit
A detection kit and technology for QT syndrome, applied in the field of human genetics and internal medicine and cardiovascular, can solve the problem of limited mutation sites of KCNH2 gene, so as to promote the research and development of innovative drugs and reduce the birth of children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0047]Embodiment 2-a kind of long QT syndrome detection kit
[0048] This embodiment provides a kit for detecting heterozygous missense variation of the human KCNH2 mutant gene c.1015A>T, and the composition of the kit is shown in Table 4 below:
[0049] Table 4 Kit Composition
[0050]
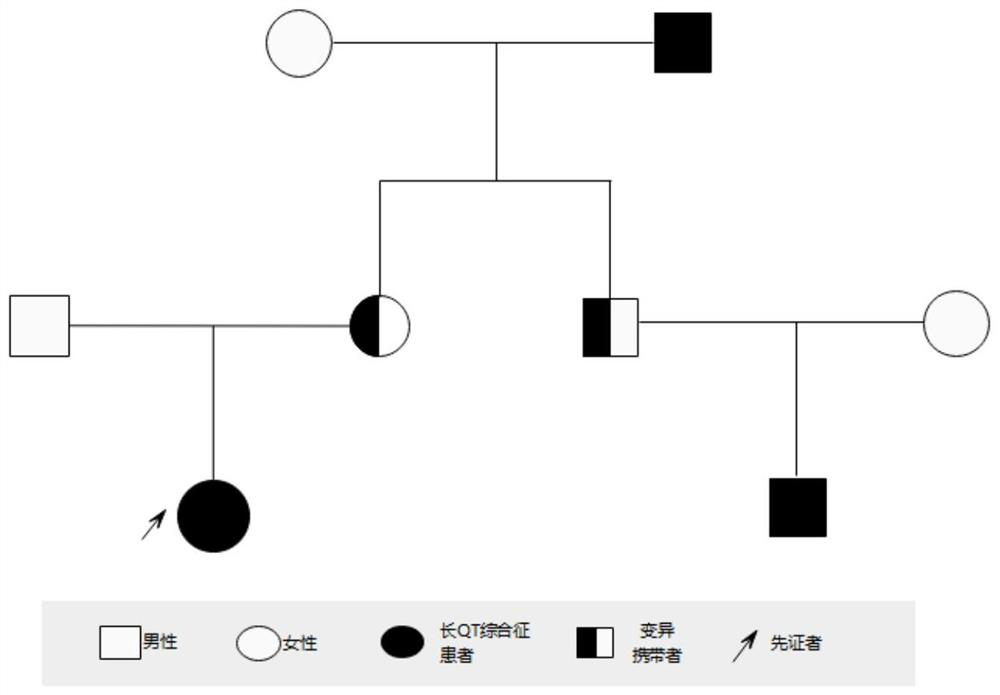
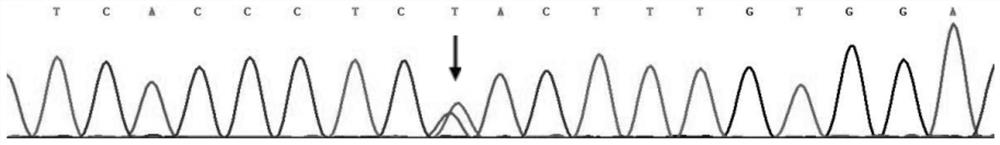
[0051] The specific steps of using this kit to screen for long QT syndrome are as follows: extract the DNA of the test subject according to the steps in Example 1, and then use the designed primer combination (SEQ ID NO:5 and SEQ ID NO:6) to carry out KCNH2 gene Amplify to obtain PCR products, and finally sequence the PCR products. The reference sequence obtained from the NCBI (https: / / www.ncbi.nlm.nih.gov / ) database is compared with the sequencing results to determine whether the subject’s KCNH2 gene carries the c.1015A>T heterozygous missense mutation, and to assist clinical Determine whether the test subject suffers from long QT syndrome type 2.
Embodiment 3
[0052] Example 3 - Mutation verification for normal people outside the family line
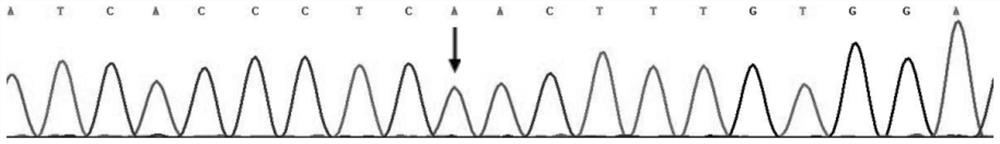
[0053] Referring to the method of Example 1, 463 cases of unrelated normal people of the same race (ie, normal people outside the family) were tested for the c.1015A>T mutation site of the KCNH2 gene, and the mutation was not detected.
[0054] Based on the above results, and based on the c.1015A>T heterozygous missense mutation of the KCNH2 gene, the protein encoded by the KCNH2 gene will undergo p.Asn339Tyr changes, and the KCNH2 gene is a known pathogenic gene of long QT syndrome. It is proved that the c.1015A>T heterozygous missense variant of KCNH2 gene is the pathogenic variant of long QT syndrome.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com