Clinical test method for tumor pharmacology
A clinical trial and pharmacology technology, applied in the field of tumor pharmacology clinical trials, can solve the problems of inability to post-process test data, not provide a network cloud platform, low efficiency, etc., and achieve the effect of saving organizational management time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
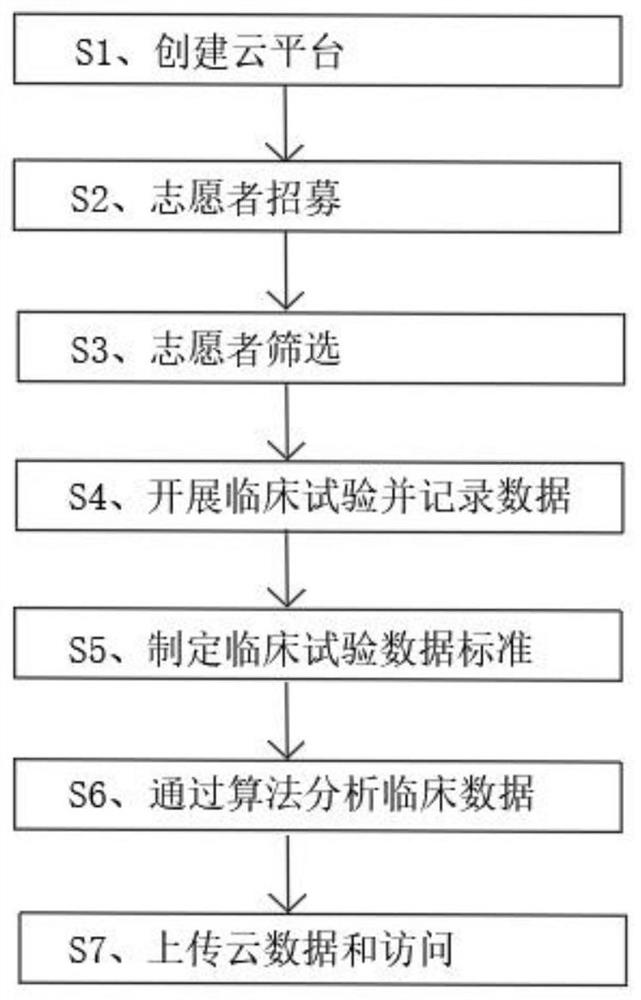
[0027] refer to figure 1 , a tumor pharmacology clinical trial method, comprising the following steps:
[0028] S1. Create a cloud platform: the cloud platform includes a data layer, the data layer includes a cloud database cluster; a service layer, the service layer includes a service platform corresponding to each user type; a clinical trial platform portal, the clinical trial platform portal Connected to the service layer through the Internet, the clinical trial platform portal can return the corresponding service platform in the service layer based on the type of visiting user;
[0029] S2. Volunteer recruitment: use the volunteer recruitment module to recruit volunteers, the volunteer recruitment module is connected to the cloud database cluster in communication, and the volunteer recruitment module is used to provide volunteer information according to the cloud database cluster issue recruitment invitations;
[0030] S3. Volunteer screening: use the volunteer screening...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com