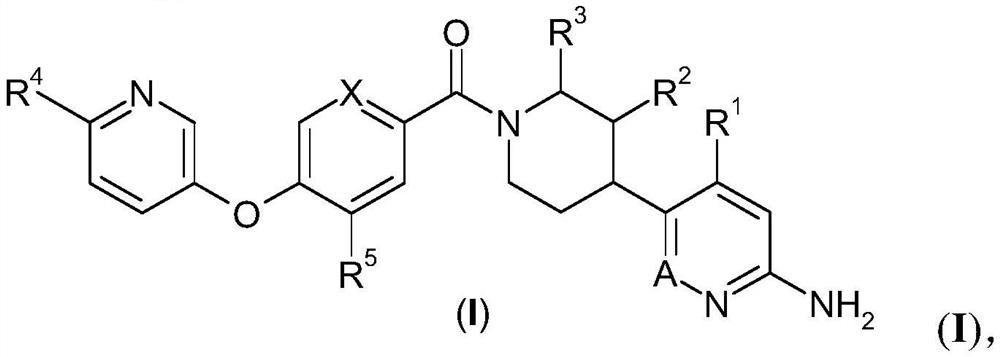
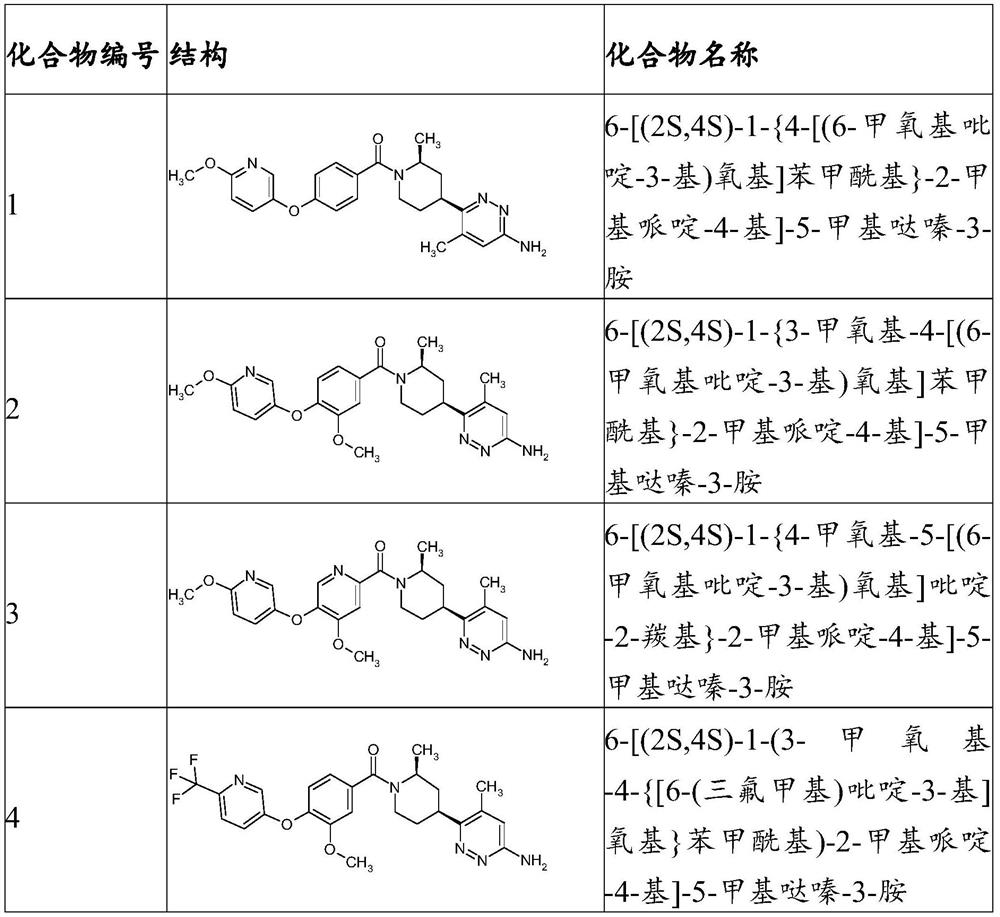
Inhibitors of trpc6
A CH2, -CH3 technology, applied in the field of TRPC6 inhibitors, can solve problems such as unclear mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0456] 6-[(2S,4S)-1-{4-[(6-methoxypyridin-3-yl)oxy]benzoyl}-2-methylpiperidin-4-yl]-5-methyl pyridazin-3-amine trifluoroacetate
[0457]
[0458] 4-[(6-Methoxypyridin-3-yl)oxy]benzoic acid (23.8 mg; 0.10 mmol), 5-methyl-6-[(2S,4S)- 2-Methylpiperidin-4-yl]pyridazin-3-amine (20.0 mg; 0.10 mmol) and DIPEA (68.4 μL; 0.40 mmol) were stirred at room temperature and HATU (36.9 mg; 0.10 mmol) was added. After stirring overnight, the reaction mixture was purified by RP-HPLC (ACN / water+TFA).
[0459] Yield: 33.8 mg (64%) ESI-MS: m / z=434 [M+H] + R t (HPLC): 0.58 (Method 11)
Embodiment 2
[0461] 6-[(2S,4S)-1-{3-methoxy-4-[(6-methoxypyridin-3-yl)oxy]benzoyl}-2-methylpiperidine-4- yl]-5-methylpyridazin-3-amine
[0462]
[0463] 3-Methoxy-4-[(6-methoxypyridin-3-yl)oxy]benzoic acid (20.0 mg; 0.07 mmol), HATU (30.0 mg; 0.08 mmol) in DMF (2 mL) and DIPEA (60.0 μL; 0.35 mmol) were stirred at room temperature for 30 minutes. 5-Methyl-6-[(2S,4S)-2-methylpiperidin-4-yl]pyridazin-3-amine (15.0 mg; 0.07 mmol) was added. After stirring for 3 hours, the reaction mixture was passed through RP-HPLC (ACN / water+NH 3 )purification.
[0464] Yield: 6.4 mg (19%) ESI-MS: m / z=464 [M+H] + R t (HPLC): 0.81 (Method 1)
Embodiment 3
[0466] 6-[(2S,4S)-1-{4-methoxy-5-[(6-methoxypyridin-3-yl)oxy]pyridine-2-carbonyl}-2-methylpiperidine- 4-yl]-5-methylpyridazin-3-amine
[0467]
[0468] 4-Methoxy-5-[(6-methoxypyridin-3-yl)oxy]pyridine-2-carboxylic acid (20.0 mg; 0.07 mmol), HATU (30.0 mg; 0.08 mmol) and DIPEA (60.0 [mu]L; 0.35 mmol) were stirred at room temperature for 30 minutes. 5-Methyl-6-[(2S,4S)-2-methylpiperidin-4-yl]pyridazin-3-amine (15.0 mg; 0.07 mmol) was added. After stirring overnight at room temperature, the reaction mixture was passed through RP-HPLC (ACN / water+NH 3 )purification.
[0469] Yield: 2.0 mg (6%) ESI-MS: m / z=465 [M+H] + R t (HPLC): 0.76 (Method 1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com