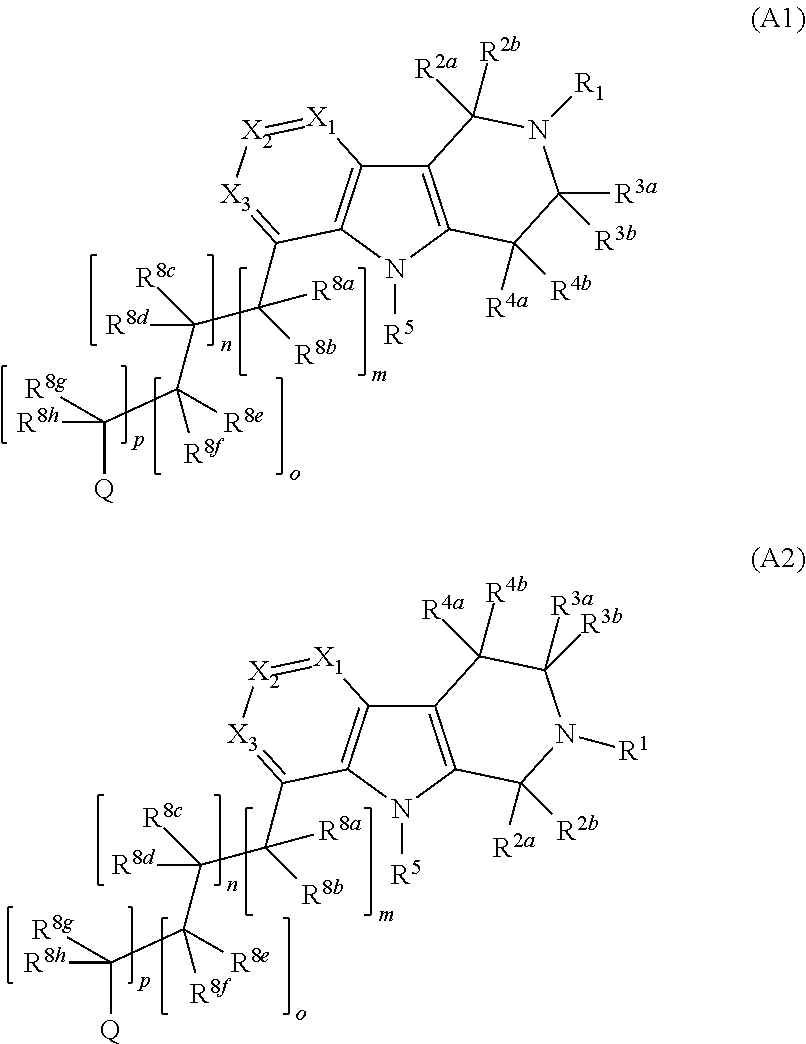
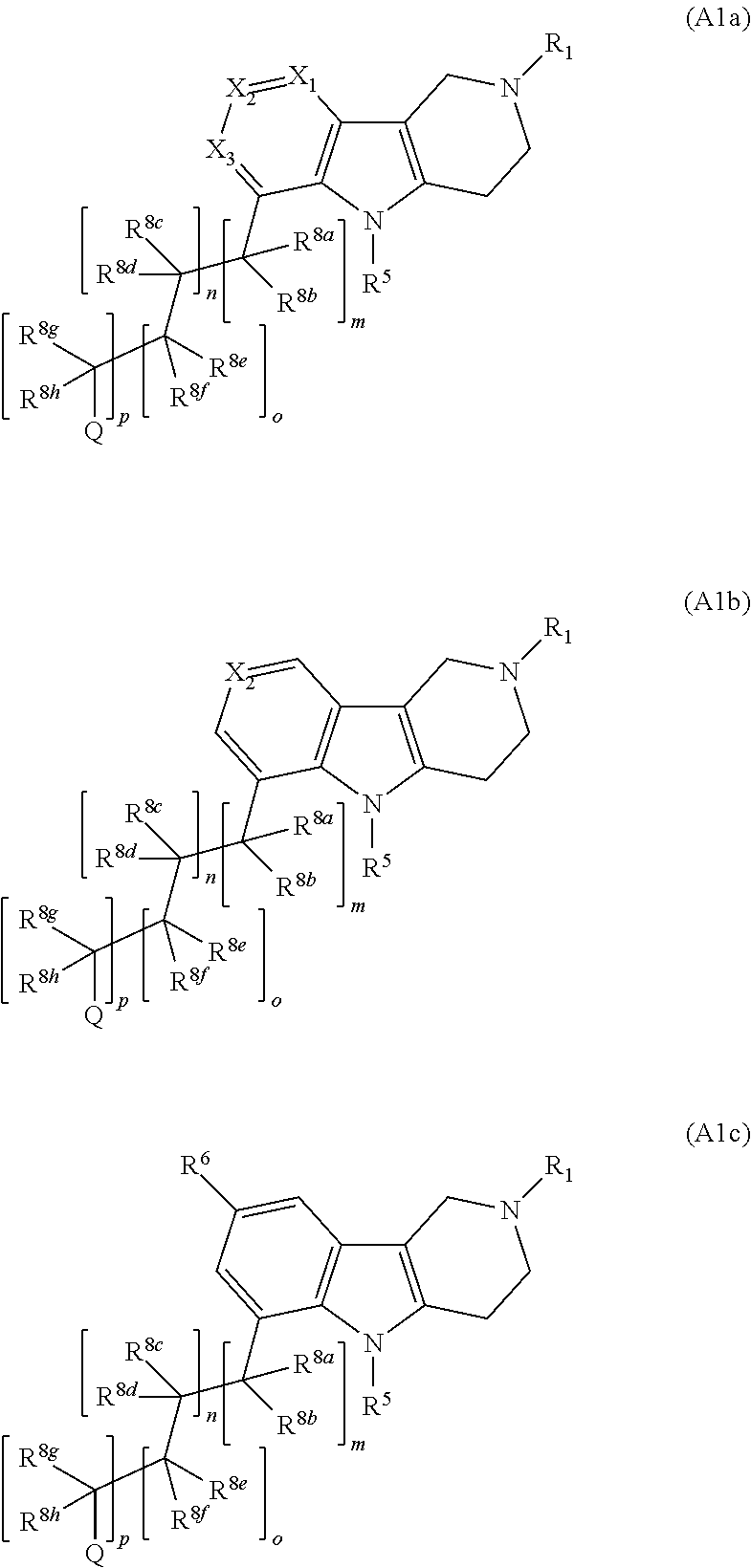
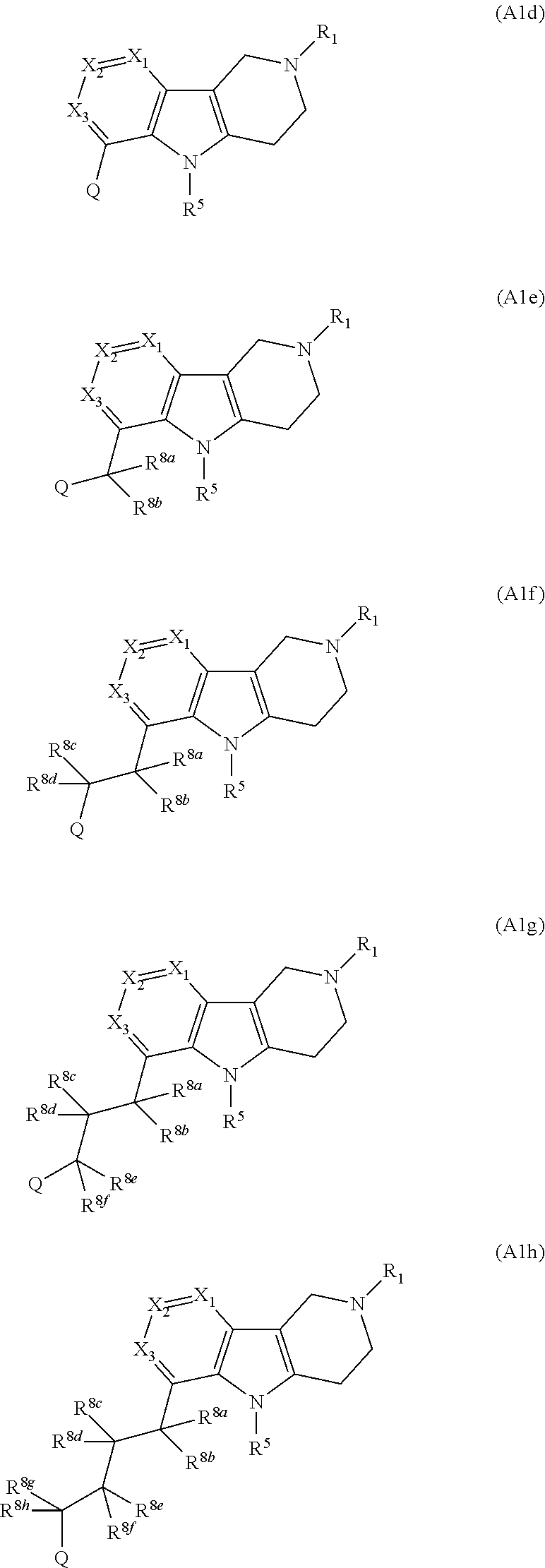
Compounds and methods of treating hypertension
a hypertension and compound technology, applied in the field of compound and method of treating hypertension, can solve the problems of increasing urine sodium content and/or urine volume, increasing renal blood flow, and increasing urine sodium content, so as to increase renal blood flow, reduce sodium reabsorption, and increase renal blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example b1
Determination of the Ability of Compounds of the Invention to Bind an Adrenergic Receptor
Adrenergic α2B
[0551]To evaluate in radioligand binding assays the activity of compounds of the invention, human recombinant adrenergic α2B receptor expressed in Chinese hamster ovary (CHO) K1 cells (Uhlen, S. et al, Eur. J. Pharmacol. 343(1):93, 1998) in a modified Tris-HCl buffer (50 mM Tris-HCl, pH 7.4, 12.5 mM MgCl2, 1 mM EDTA, 0.2% BSA) was used. Compounds of the invention were incubated with 2.5 nM [3H]Rauwolscine for 60 min at 25° C. Non-specific binding was estimated in the presence of 10 μM Prazosin. Receptor proteins were filtered and washed, the filters were then counted to determine [3H]Rauwolscine specifically bound. Compounds were screened at 1 μM or lower, using 1% DMSO as vehicle. Compounds of the invention were tested in this biochemical assay and percent inhibition of specific binding was determined. Biochemical assay results are presented as the percent inhibition of specific ...
example b2
Functional Activity on Recombinant Adrenergic α1B, Adrenergic α2A Adrenergic α2B and Adrenergic α1D Receptors Using Aequorin and GTPγS Functional Assays
[0555]To study the functional activity of compounds of the invention on the human recombinant adrenergic α2B, adrenergic α2A, adrenergic α1B or adrenergic α1D with Aequorin functional assays and on the human recombinant adrenergic α2B receptor with GTPγS assay, CHO-K1 cell lines expressing adrenergic α2B, adrenergic α2A, adrenergic α1B or adrenergic α1D recombinant receptor, mitochondrial apoaequorin and Gα16 are used for the Aequorin assay. CHO-K1 cell line expressing the recombinant α2B receptor is amplified to prepare membranes used for the GTPγS assay.
[0556]The following reference agonists are used as both the reference ligand in agonist mode and as the agonist that needs to be inhibited in antagonist mode.
α2BAssayα1B (aeq)α1D (aeq)α2A (aeq)α2B (aeq)(GTPgS)AgonistCirazolineCirazolineUK 14304Oxyme-Guanfacineligandtazoline
[0557]Aeq...
example b3
Cell Culture and Cell Viability Assay
[0568]SH-SY5Y cells cultured in DMEM / F12 media supplemented with 10% FBS were seeded in 96-well microplates at 150,000 cells / cm2. After 24 h, cells were depleted from FBS and kept in culture for 24 h before the experiment. A stock solution was prepared by dissolving the calcium ionophore 4-Br-A23187 (Calbiochem Cat. No 100107) in DMSO at 25 mM. Cells were then treated with 4-Br-A23187 (2 μM), hydrogen peroxide (300 μM) or the mitochondrial toxin rotenone (25 μM) in the presence of vehicle or Compound of the Invention for 24 h. Cell death was determined by measurements of LDH release according to the Cytotoxicity Detection KitPlus (Roche, Mannheim, Germany). Cell viability was determined by measuring the capacity of cells to metabolize MTS tetrazolium (MTS) according to the Cytotoxicity Detection KitPlus (Roche, Mannheim, Germany) and MTS reduction was assessed by the CellTiter 96® AQueous One Solution Cell Proliferation assay (Promega Corporation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com