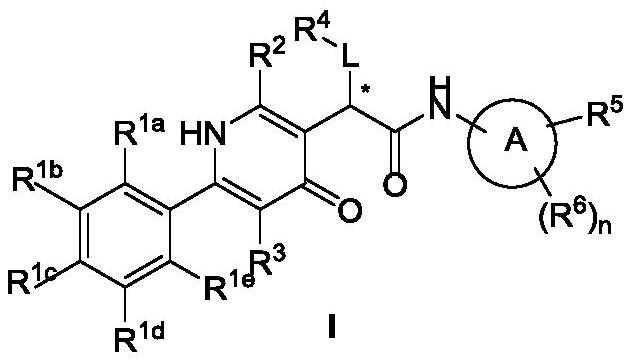
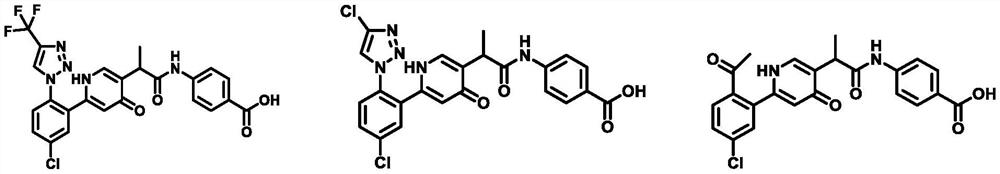
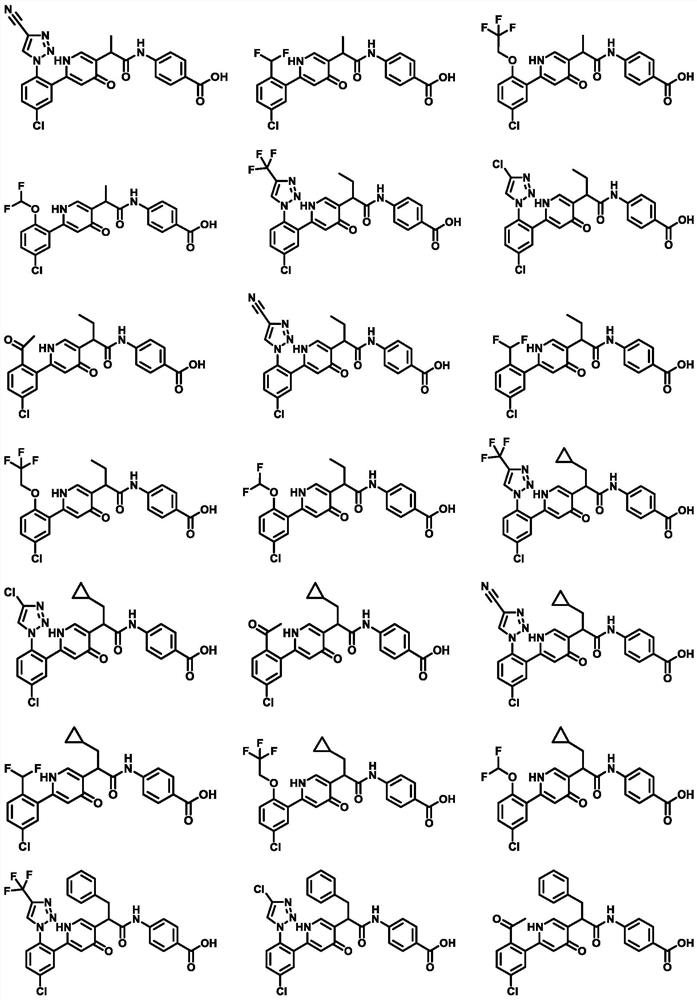
4 (1H)-pyridone compound as well as pharmaceutical composition and application thereof
A technique for pyridone and compounds, applied in the field of 4-pyridone compounds, capable of solving problems such as single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] The synthesis of embodiment 1 compound 26, 26-P1, 26-P2
[0258]
[0259] Reaction formula:
[0260]
[0261] The first step: the synthesis of compound 26-2
[0262] DAST (17.5g) was added to a solution of compound 26-1 (16g) in DCM (200mL) at 0°C, stirred at 25°C for 16 hours, washed with saturated sodium bicarbonate (100mL), extracted with DCM (100*3mL), It was concentrated under reduced pressure, and the residue was purified by silica gel column (petroleum ether) to obtain compound 26-2 (17.1 g, yield 97%).
[0263] The second step: the synthesis of compound 26-4
[0264] Compound 26-2 (2.4g), compound 26-3 (3.04g), potassium acetate (2.44g), Pd(dppf)Cl 2 (0.22g) was added to dioxane (50mL) solution, reacted at 85 degrees Celsius under the protection of reaction liquid nitrogen for 16 hours, added water (20mL) and extracted with ethyl acetate (20mL*3), the organic phase was concentrated under reduced pressure, and the residue Purification with silica gel co...
Embodiment 2
[0281] The synthesis of embodiment 2 compound 296, 296-P1, 296-P2
[0282]
[0283] Reaction formula:
[0284]
[0285] The first step: the synthesis of compound 296-6
[0286] Compound 296-4 (316mg), compound 296-5 (0.73g), sodium carbonate (0.42g), Pd(dppf)Cl 2 (0.29g) was added to a mixed solution of DME (50mL), ethanol (6mL) and water (6mL), reacted at 95 degrees Celsius for 16 hours under the protection of reaction liquid nitrogen, added water (20mL), extracted with ethyl acetate (20mL*3) , the organic phase was concentrated under reduced pressure, and the residue was purified by silica gel column (ethyl acetate / petroleum ether=1 / 5) to obtain compound 296-6 (920 mg, yield 75%).
[0287] The second step: the synthesis of compound 296-7
[0288] Compound 296-6 (2.2 g) was added to THF (40 mL) solution at 25 degrees Celsius, NaBH 4 (1.02g), methanol (20mL), the reaction solution was stirred at 40°C for 16 hours, quenched by adding 5mL of ammonium chloride to the re...
Embodiment 3
[0309] The synthesis of embodiment 3 compound 28, 28-P1, 28-P2
[0310]
[0311] Reaction formula:
[0312]
[0313] The first step: the synthesis of compound 28-2
[0314] Compound 28-1 (12g), cesium carbonate (37.8g), and sodium difluorochloroacetate (22g) were successively dissolved in a mixed solution of DMF (450mL) and water (50mL), stirred at 100°C for 16 hours, then added with water (2000mL) Extracted with ethyl acetate (200 mL*3), the organic phase was concentrated under reduced pressure, and the residue was purified by silica gel column (petroleum ether / ethyl acetate=20 / 1) to obtain compound 28-2 (11 g, yield 69%).
[0315] The second step: the synthesis of compound 28-3
[0316] Compound 28-2 (2g), bis-boron (2.4g), potassium acetate (1.9g), Pd(dppf)Cl 2 (170mg) was added to dioxane (80mL) solution, reacted at 100 degrees Celsius under the protection of reaction liquid nitrogen for 16 hours, added water (20mL) and extracted with ethyl acetate (20mL*3), the org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com