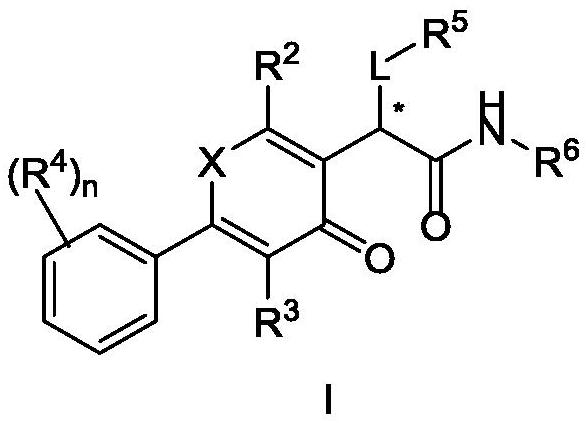
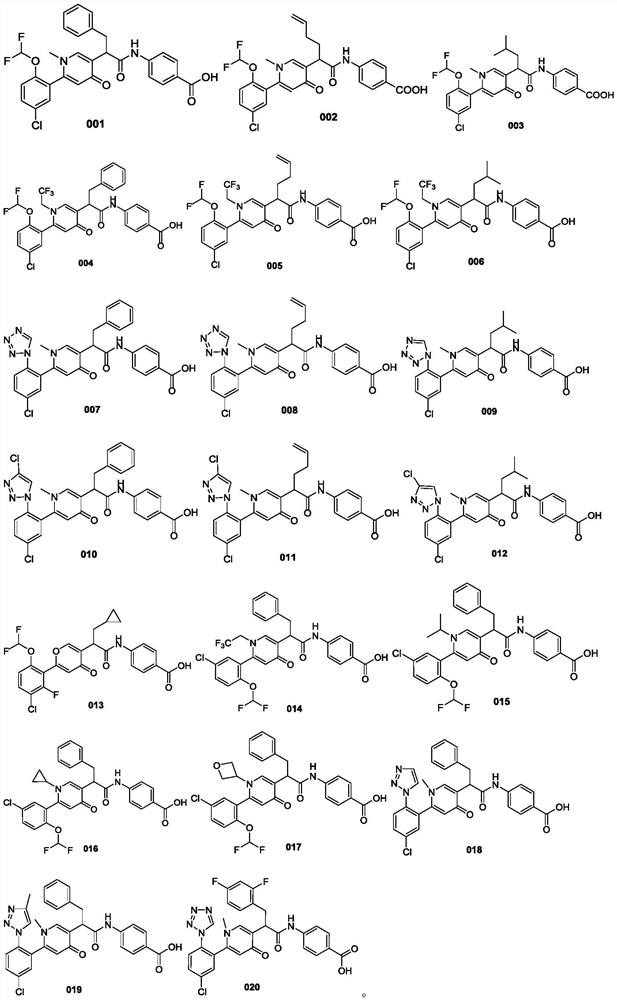
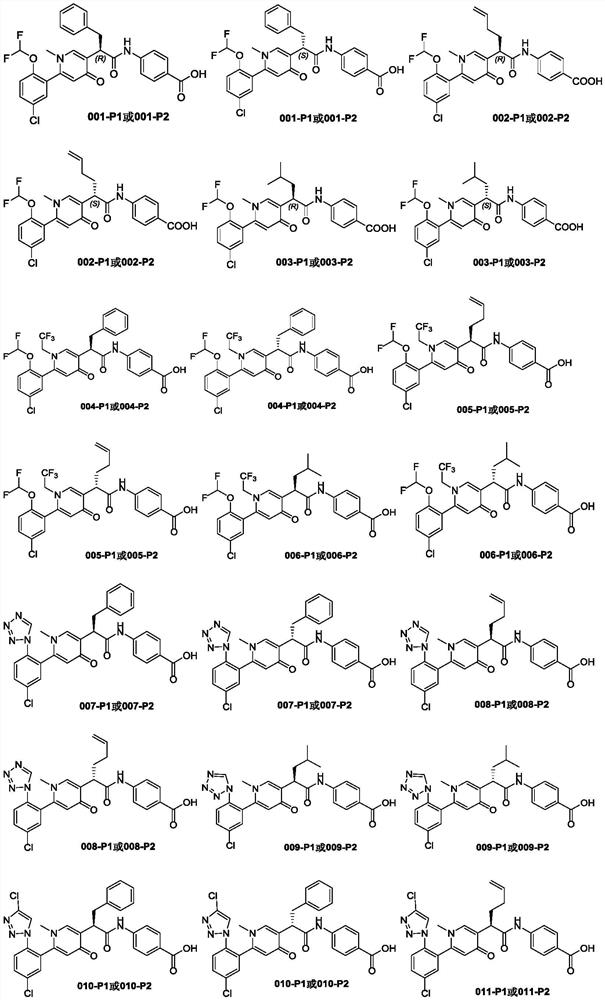
Heterocyclic compound and application thereof
A technology of heterocyclic compounds, applied in the field of heterocyclic compounds, can solve the problems of lethality, inability to protect patients from acute myocardial ischemia, low incidence of deep vein thrombosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Synthesis of target compounds 001, 001-P1, 001-P2
[0226]
[0227] Step 1: Preparation of Compound 2
[0228] Magnesium turnings (0.8 g) and a small particle of iodine were added to 20 mL of THF, benzyl bromide (2.7 g) was added dropwise at 25 degrees Celsius, and the mixture was stirred at room temperature for 2 hours. The prepared Grignard reagent was slowly added dropwise to a solution of compound 1 (3 g) in THF (20 mL) and stirred at 25 degrees Celsius for 1 hour. The reaction was quenched by adding 100 mL of saturated aqueous ammonium chloride solution, extracted with ethyl acetate (50 mL×3), the mixed organic phase was washed with concentrated brine (50 mL×2), dried and concentrated through the column, ethyl acetate: petroleum ether=1:5 A white solid product (ie compound 2, 2.5 g, 78% yield) was obtained. MS m / z (ESI): 404 [M+1].
[0229] Step 2: Preparation of Compound 3
[0230] Compound 2 (2.5 g) and p-toluenesulfonylmethylisonitrile (2.4 g) were dissol...
Embodiment 2
[0245]Synthesis of target compounds 002-P1 and 002-P2
[0246]
[0247] Step 1: Preparation of Compound 3
[0248] Compound 1 (41.5 g) was added to a 1000 mL single-neck flask, and the solvent DMF (400 mL) and water (40 mL) were added. Sodium 2-chloro-2,2-difluoroacetate (39.6 g) and potassium carbonate (41.5 g) were added to the reaction system, and the reaction was heated to 100 degrees Celsius for 16 hours. After the reaction, add water (300 mL) to dilute, extract twice with ethyl acetate (500 mL), wash once with brine (200 mL), and evaporate the organic phase to dryness at 45°C. Silica gel was added and the sample was mixed, and purified by column (PE) to obtain a yellow oily liquid (ie, compound 3, 46 g, yield 80%). MS m / z (GCMS): 256.
[0249] Step 2: Preparation of Compound 4
[0250] Compound 3 (46g) was added to a 1000mL single-necked bottle, a solvent dioxane (500mL) was added, pinacol biboronate (49.9g), potassium acetate (35g), Pd(dppf)Cl were added. 2 DCM...
Embodiment 3
[0278] Synthesis of target compounds 003-P1 and 003-P2
[0279]
[0280] Step 1: Preparation of Compound 2
[0281] Compound 1 (3.72g) was added to a 250mL single-neck bottle, solvent THF (50mL) was added, liquid nitrogen was cooled to -78 degrees Celsius under nitrogen protection, 2-methylpropylmagnesium bromide (2M in THF, 15mL) was slowly added Into the reaction system, the reaction mixture was naturally warmed to room temperature, and the reaction was carried out for a total of 4 hours. After the reaction, add water (50mL) to dilute, extract twice with ethyl acetate (100mL), wash once with brine (50mL), evaporate the organic phase to dryness at 45 degrees Celsius to obtain a yellow oily product (i.e. compound 2, 3.5g, yield: 90% ). MS m / z (ESI): 370 [M+1].
[0282] Step 2: Preparation of Compound 3
[0283]Compound 2 (3.7 g) was added to a 100 mL single-neck flask, solvent THF (50 mL), compound 9 (3.9 g) and t-BuOK (3.36 g) were added, and the reaction was carried o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com