An electronic inspection method and system for clinical trials
A clinical trial and electronic technology, applied in the field of clinical trial inspection, can solve the problems of not being able to inspect the project operation process together, staying in the stage of manual sorting and offline operation, etc., so as to improve the level of project quality management and promote scientific sequential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
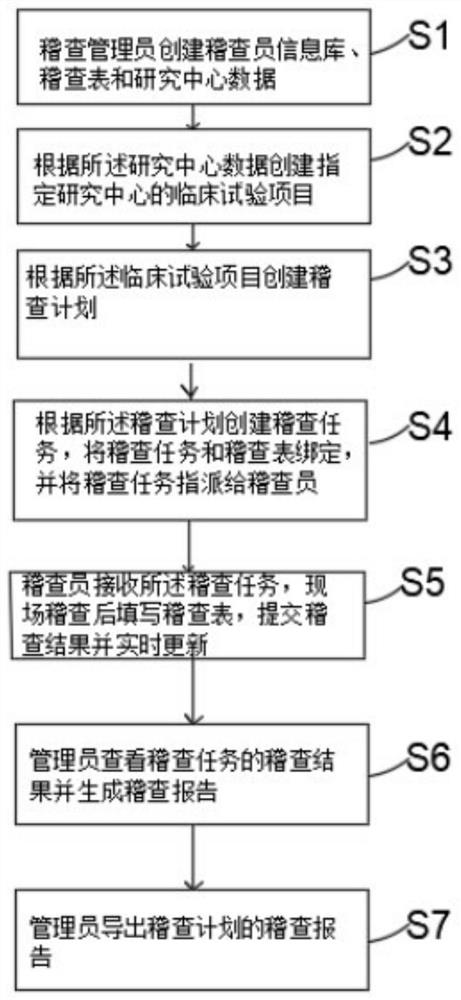
[0078] Such as figure 1 As shown, the present invention provides an electronic inspection method and system for clinical trials, which has the advantage of higher efficiency.
[0079] In order to achieve the above object, the embodiment adopted in the present invention is: an electronic inspection method for clinical trials, comprising the following steps:
[0080] S1. The audit administrator creates the auditor information database, audit form and research center data;
[0081] S2. Create a clinical trial project for a designated research center based on the data of the research center;
[0082] S3. Create an audit plan according to the clinical trial project;
[0083] S4. Create an audit task according to the audit plan, bind the audit task to the audit table, and assign the audit task to the auditor;
[0084] S5. The auditor receives the audit task, fills in the audit form after the on-site audit, submits the audit result and updates it in real time;
[0085] S6. The ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com