Pyrazolopyridine compounds for ire1 inhibition
A compound and solvate technology, applied in the field of pyrazolopyridine compounds for IRE1 inhibition, can solve problems such as instability of ER protein folding mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0427] The invention will now be described with reference to the following examples. These examples are provided for illustrative purposes only, and the invention is not limited to these examples but encompasses all variations apparent as a result of the teaching provided herein.
[0428] Materials and methods
[0429] General Experimental Details
[0430] Unless otherwise stated, reactions were not performed under an inert atmosphere and all solvents and commercial reagents were used as received.
[0431] Purification by chromatography refers to the use of Companion purification system or Biotage SP1 purification system for purification. In use In the case of SPE Si II cartridges (cartridges) for product purification, 'Isolute SPE Si cartridges' refer to prepacked polypropylene columns containing unbonded reactive silica gel, with irregular particles, with an average particle size of 50 μm, and labeled porosity Fractions containing the desired product (identified by...
Embodiment approach
[0920] The following exemplary embodiments are provided, the numbering of which should not be construed as specifying a level of importance:
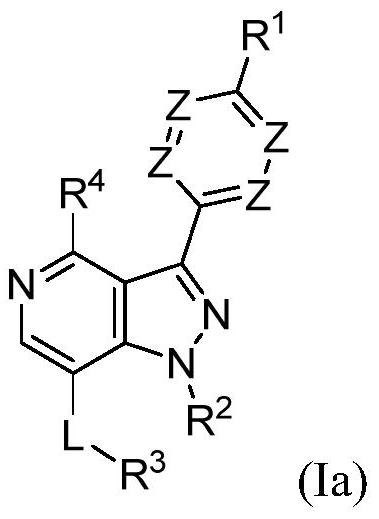
[0921] Embodiment 1 provides a compound of formula (Ia), or a salt, solvate, enantiomer, diastereoisomer, isotope or tautomer thereof: in:
[0922] R 1 Yes
[0923] R 2 selected from H, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, CF 3 、CHF 2 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and 1-methylcyclopropyl;
[0924] L is the key;
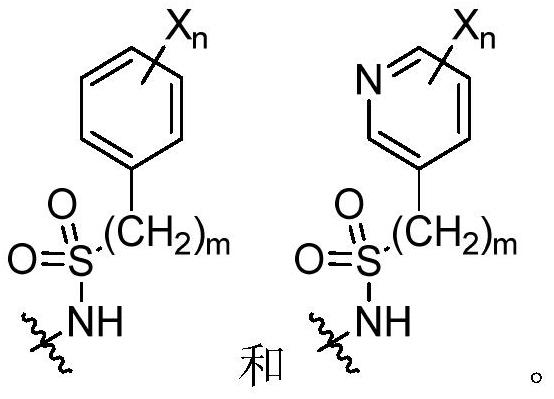
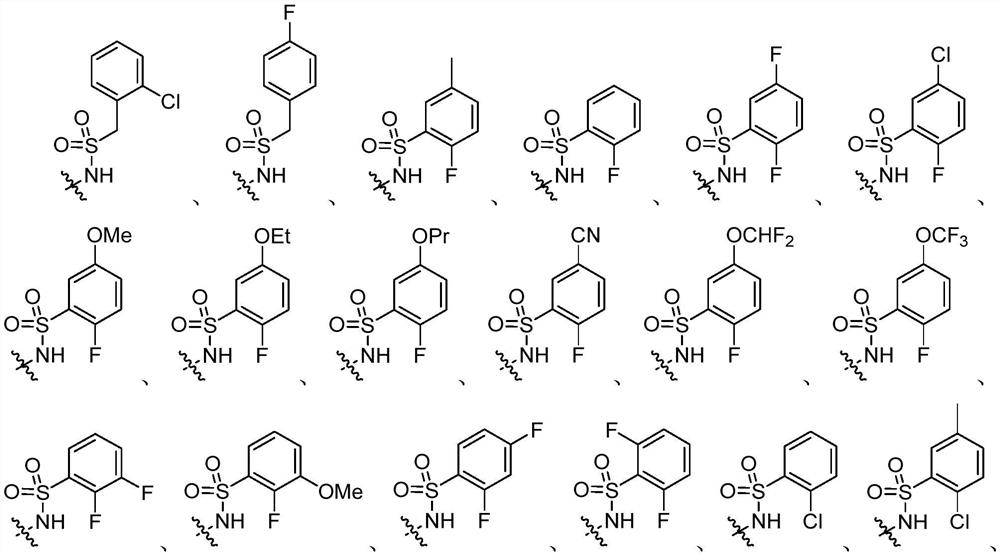
[0925] R 3 selected from optionally substituted C 3 -C 8 Cycloalkyl, optionally substituted C 3 -C 8 Cycloalkenyl, optionally substituted C 3 -C 8 Heterocycloalkyl and optionally substituted C 2 -C 8 cycloheteroalkenyl;
[0926] R 4 Yes-NH 2 ;
[0927] 0-3 Z's are N and the remaining Z's are independently CR 5 ;
[0928] each R 5 independently selected from halogen, -OH, optionally substituted C 1 -C 6 Alkyl and optiona...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com