Tissue defect closing instrument
A device and tissue technology, applied in the field of cardiac tissue defect closure device, can solve the problems of human tissue damage, poor occlusion stability, poor anchoring effect of occluder, etc., to reduce stimulation damage, reduce shear area, good The effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
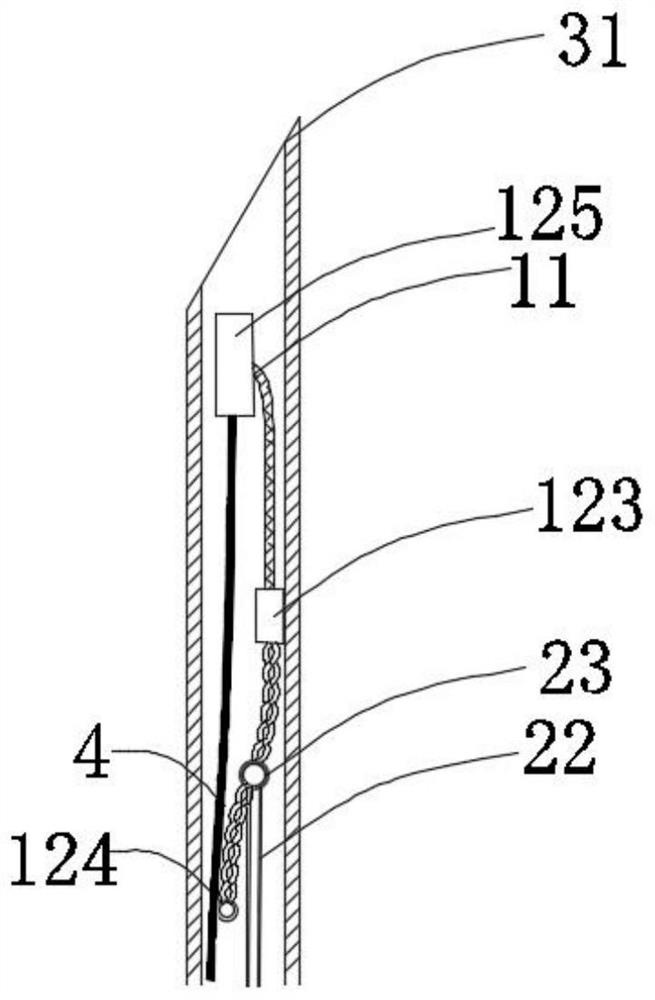
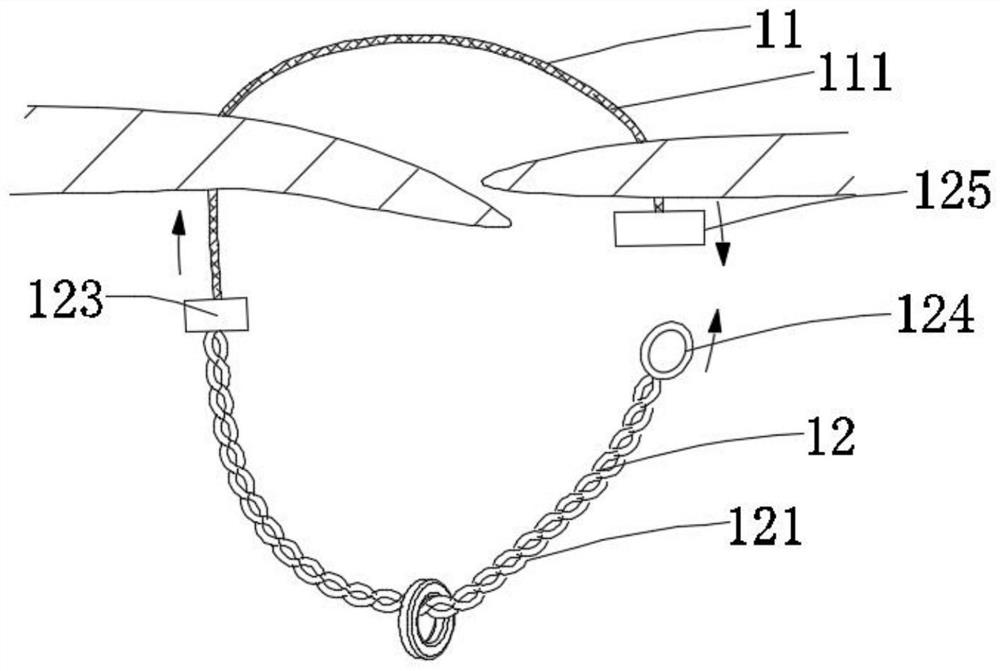
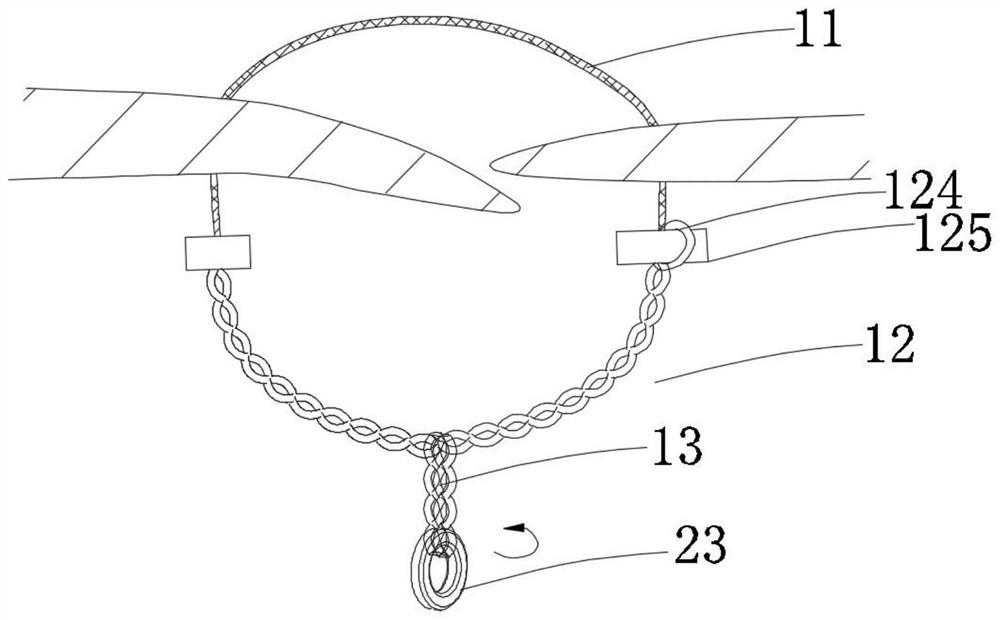
[0133] In this example, if Figure 1a~1c As shown, the closing device includes a closing unit and a release unit 13. The closing unit includes a connecting unit 11 and a locking unit 12 connected to the connecting unit 11. The locking unit 12 is located at the proximal end region of the connecting unit 11. The release unit 13 is located in the proximal region of the locking unit 12; the connecting unit 11 only includes one connecting piece 111; the locking unit 12 includes two locking pieces 121; the unlocking unit 13 is operated to drive the locking piece 121 to rotate , and the connecting unit 11 is tightened, the locking parts 121 are close to each other and realize self-locking locking, thereby realizing the closure of the tissue defect, and the closing unit part is connected with the tissue; the locking unit 12 and the release unit 13 are of an integrated structure, When the locking member 121 realizes self-locking and locking, the release unit 13 is further operated, so ...
Embodiment 2
[0160] The difference from Example 1 is:
[0161] In this example, if Figure 3b As shown, the closing unit includes a connecting unit 11 and one or more fixing members 16 located at the distal end region of the connecting unit 11; area, and connected with the connecting member 111, part or all of the fixing member 16 is attached or anchored to the inside of the target tissue or the outer surface of the distal end.
[0162] In this example, if Figure 3b As shown, when the connecting unit 11 located in the proximal region of the tissue defect is in an open state, the locking unit 12 and the connecting unit 11 are connected through the fixed connection structure 123 .
[0163] In this embodiment, the locking unit 12 includes two locking elements 121 , and each locking element 121 includes three wires.
[0164] In this embodiment, the fixing part 16 and the connecting part 111 are stretched in the tissue defect area, and the posture adjustment structure 14 adjusts the angle b...
Embodiment 3
[0190] The difference with embodiment one is:
[0191] In this example, if Figures 11a-11d As shown, the connecting unit 11 is a polymer wire, the locking unit 12 is a soft metal wire, and the locking unit 12 includes two locking parts 121, and each locking part 121 is connected by gluing, mechanical connection, welding, etc. On the connector and twisted with the connector 111 to form.
[0192] In this embodiment, the release unit 13 is operated so that the auxiliary breaking structure 8 drives the locking member 121 to rotate, and the locking members 121 are close to each other and realize self-locking locking, thereby realizing the closure of the tissue defect ; when the locking member 121 achieves self-locking and locking, the release unit 13 is further operated, and the auxiliary breaking structure 8 is further rotated, so that the auxiliary breaking structure 8 is broken and separated from the locking member 121, Such as Figures 11a-11d As shown, the connecting piece...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com